BioChemistry > QUESTIONS & ANSWERS > Virginia Commonwealth University BIOC 403 Summer Test2 Key Biochemistry 403 Exam #2 (All)
Virginia Commonwealth University BIOC 403 Summer Test2 Key Biochemistry 403 Exam #2
Document Content and Description Below
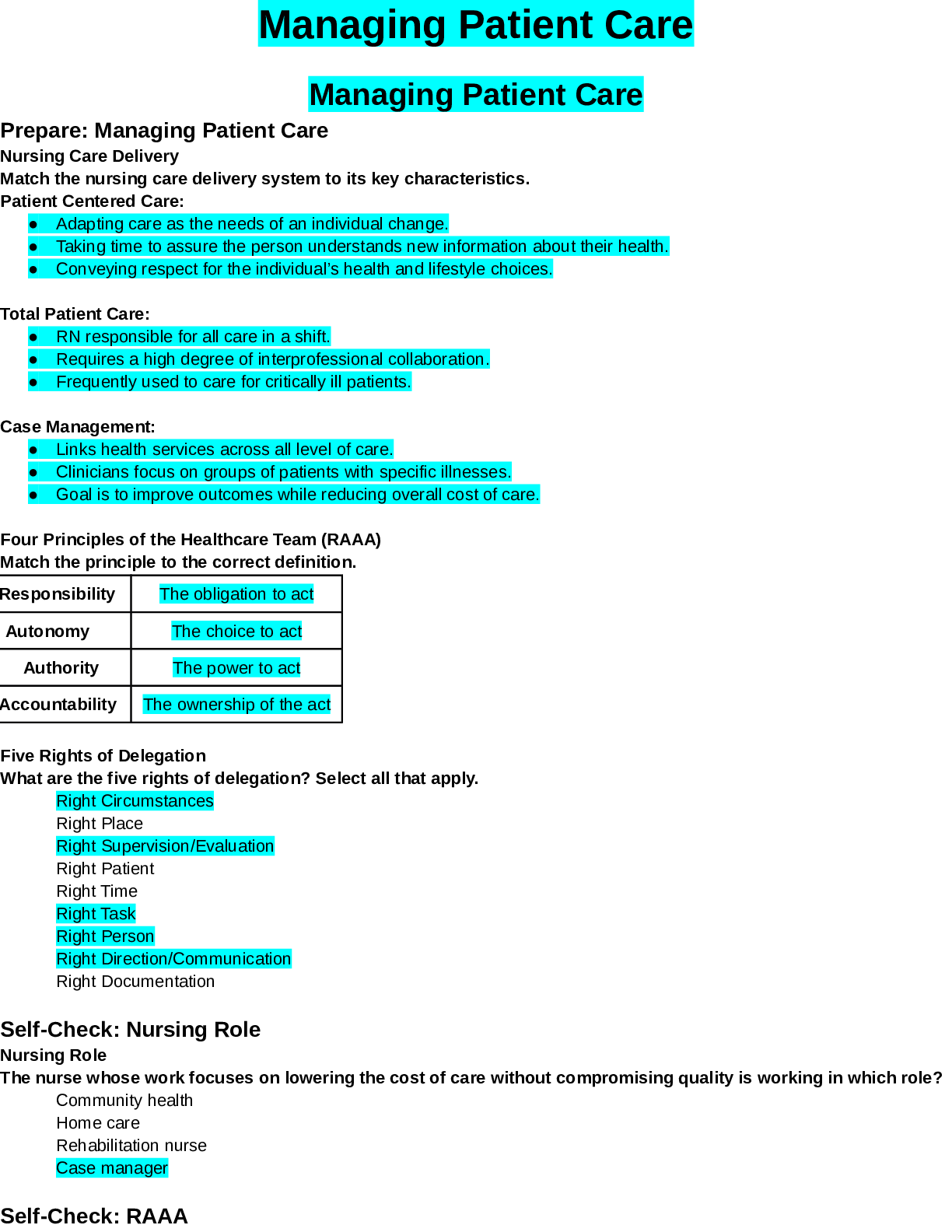
Virginia Commonwealth University BIOC 403 403 Summer Test2 Key Biochemistry 403 Exam #2 Name______________Key__________________ I pledge that I have neither given nor received aid on this test.... __________________________________ Signed Multiple Choice (2 points each) 1. The demonstration that RNA can have catalytic activity suggests that: a. DNA may be more catalytic than RNA b. Proteins do not catalyze reactions c. An RNA world led to life d. RNA cannot contain genetic information e. None of the above 2. The catalytically active complex of ___________ and _________ is called the _______________. a. apoenzyme; holoenzyme; cofactor b. cofactor; holoenzyme; apoenzyme c. holoenzyme; cofactor; apoenzyme d. apoenzyme; cofactor; holoenzyme e. none are true 3. In the Lineweaver-Burk double reciprocal plot, which of the following is NOT a true representation? a. slope = Km/Vmax b. y-intercept is -1/Vmax c. x-intercept is - 1/Km d. y = 1/V e. x = 1/[S] 4. All are true for inhibitor I if it is a competitive inhibitor EXCEPT: a. EI is not converted to P b. I is structurally similar to the substrate. c. I binds to a site other than the active site. d. I decreases vmax. e. I does not change vmax. 5. The catalytic mechanism below is an example of: a. covalent nucleophilic catalysis. b. covalent electrophilic catalysis. c. specific base catalysis. d. general base catalysis. e. low barrier hydrogen bond catalysis. 6. In the hydrolysis of p-nitrophenylacetate by chymotrypsin all of the following are correct EXCEPT: a. the second product is acetic acid. b. an acetyl-enzyme intermediate forms during the mechanism. c. attack of a water molecule on the acyl-enzyme intermediate yields the second product. d. the active site contains a serine residue, a histidine, and an aspartate residue. e. none, all are true. 7. All are characteristics of ordered single-displacement reactions EXCEPT: a. Lineweaver-Burk plots with lines that intersect to the left of the 1/v axis. b. a chemically modified enzyme-intermediate. c. the lack of any exchange reaction activity. d. Ping pong kinetics. e. Ternary enzyme –substrate complex formation 8. The slowest method for influencing enzymatic activity is usually by: a. Increasing enzyme amount. b. covalent modification. c. phosphorylation. d. activation of a zymogen. e. allosteric regulation. 9. All are characteristic of allosteric enzymes EXCEPT: a. Effectors may show stimulatory or inhibitory activity. b. They have multiple subunits. c. They obey Michaelis-Menten kinetics. d. The regulatory effect is by altering conformation and interaction of subunits. e. Binding one subunit impacts binding of substrate to other subunits. 10. All are true for the enzyme-transition state complex EXCEPT: a. It is designated as EX‡. b. The enzyme stabilizes the transition-state complex more than it stabilizes the substrate complex. c. The enzyme is “designed” to bind the transition-state structure more tightly than the substrate or product. d. The energy barrier between ES and EX‡ is less than the energy barrier between S and X‡. e. All are true. 11. Because the enzymatic reaction rate is determined by the difference in energy between ES and _____, the tighter E binds to s, the ________ the rate of reaction. a. S; higher b. P; lower c. EX‡; lower d. EX‡; higher e. S; lower 12. Proteins generally fold in the following order: a. Hydrophobic collapse, 2o structure interactions, 2o structure formation b. 2o structure formation, 2o structure interaction, ionic collapse c. 2o structure formation, 2o structure interaction, hydrophobic collapse d. 2o structure interaction, ionic collapse, 3o structure interactions e. None of the above 13. Transition-state analogs are: a. approximations of the transition state that bind more tightly than the substrate. b. compounds that compete for the active site, but are not necessarily very similar to the substrate. c. stable molecules that can not be expected to resemble the true transition state too closely. d. stable chemically and structurally similar molecules to the transition state. e. all of the above. 14. Most covalent catalysis is carried out by enzymes using a: a. allosteric mechanism. b. sequential bisubstrate kinetic mechanism. c. random bisubstrate kinetic mechanism. d. ping-pong kinetic mechanism. e. none of the above. 15. Because the pKa is near 7, _____________ side-chains are often involved in general acid-base catalysis. a. cysteine b. aspartate c. glutamate d. lysine e. histidine 16. If the rate constant for the enzyme catalyzed reaction is 4 X 104/sec and the rate constant for the uncatalyzed reaction is 4 X 10-4/sec, the catalytic power of the enzyme is: a. 4 X 108 b. 2.6 X 107 c. 1 X 10-8 d. 1 X 108 e. 2 X 10-1 17. Amylose and amylopectin share all of the following characteristics except: a. Both D glucose polymers b. Both have a1-4 linkages c. Both are starches d. Both are found in plants e. Both are branched 18. Peptidoglycan in gram positive and gram negative cells share all of the following characteristics except: a. Both have 5 Gly residues crosslinking strands b. Both have attached tetrapeptides c. Both contain amino acids not found in proteins d. Both contain polysaccharides e. Both are heteropolysaccharides 19. N linked polysaccharides are usually attached to proteins at ________ residues. a. Asn b. Asp c. Arg d. Lys e. None of the above 20. A plot of vo vs [s] is (a) __________________ for a regulatory enzyme. a. Rectangular hyperbola b. Straight line c. Sigmoidal d. Circular e. None of the above 21. For each sugar indicate stereochemistry (D or l) and the total number of possible stereoisomers (10 p[oints). D D L D L 2 4 8 2 32 22. A. (8 points) What are the natural negative and positive effectors for the enzyme glycogen phosphorylase (phosphorylase b form)? Positive: ADP Negative: ATP, glucose, glucose-6-Pi B. (12 points). [Glucose] drops to a dangerously low level in the bloodstream. What physical changes occur with glycogen phosphorylase enzyme? How is the activity of the enzyme affected? What are the effectors now? Glycogen phosphorylase is phosphorylated in response to low [glucose] to make glycogen phosphorylase a. GP a is constitutively active. The only effector is then glucose (negative). 23. (10 points) a. What form of reversible inhibition is depicted below? b. Where on an enzyme does the inhibitor bind? c. To what form(s) of the enzyme does it bind? d. What is the inhibitors effect on the Km and Vmax of the enzyme? a. Noncompetitive b. Inhibitor binds away from active site c. Inhibitor binds both ES complex and free E. d. Km does not change, Vmax decreases. 24. (10points) Enzyme X has rate constants of k1= 2x107 sec-1, k2=8x103sec-1 and k-1= 1x102 sec-1 and a Vmax of 50 umol/ml sec. What is the velocity of the catalyzed reaction when [S] = 1mM? Km = k-1 + k2/k1= 4.05 X10-4M V=Vmax [S]/Km + [S] V= (50umol/ml sec) (1 x10-3M)/ (4.05X10-4M) + (1 X 10-3M) V=35.59 umol/ml sec 25. (10 points) Draw the mechanism for the first stage of peptide cleavage by chymotrypsin. See book [Show More]
Last updated: 2 years ago
Preview 1 out of 6 pages
.png)
Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$8.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Aug 02, 2022
Number of pages
6
Written in
Additional information
This document has been written for:
Uploaded
Aug 02, 2022
Downloads
0
Views
94
.png)











.png)

