NACE CP2 exam 2022/2023 with 100% correct answers
Document Content and Description Below
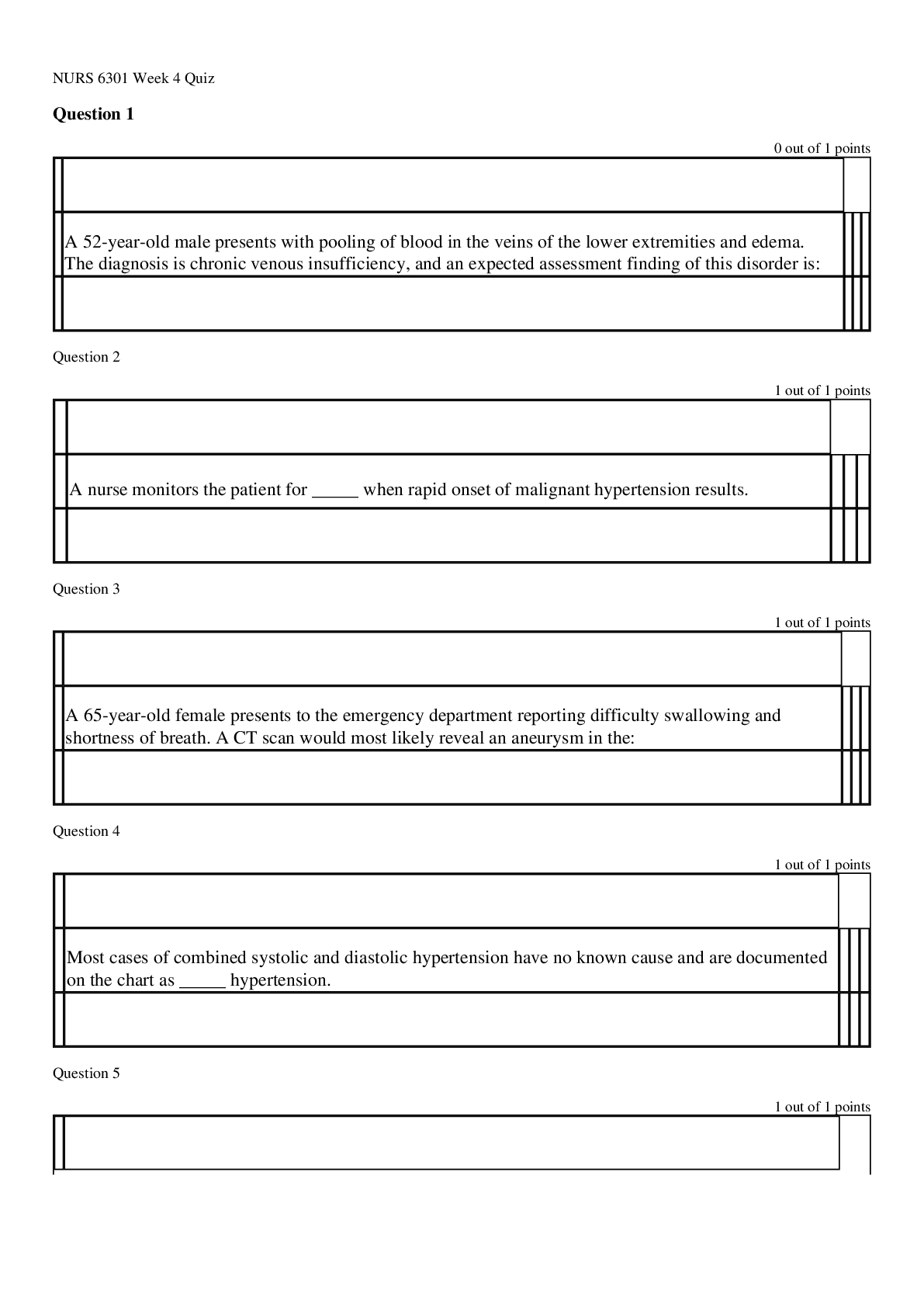
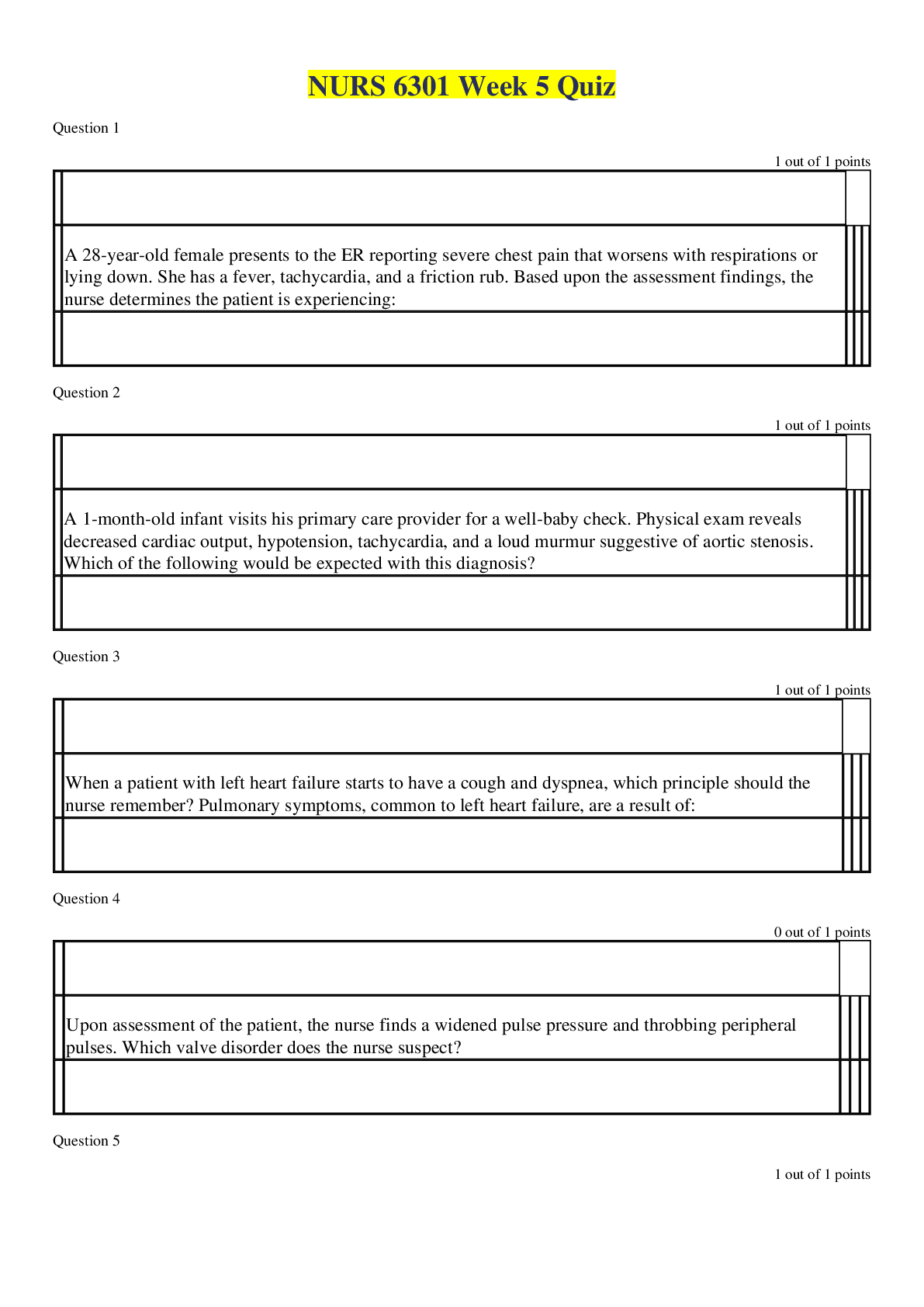
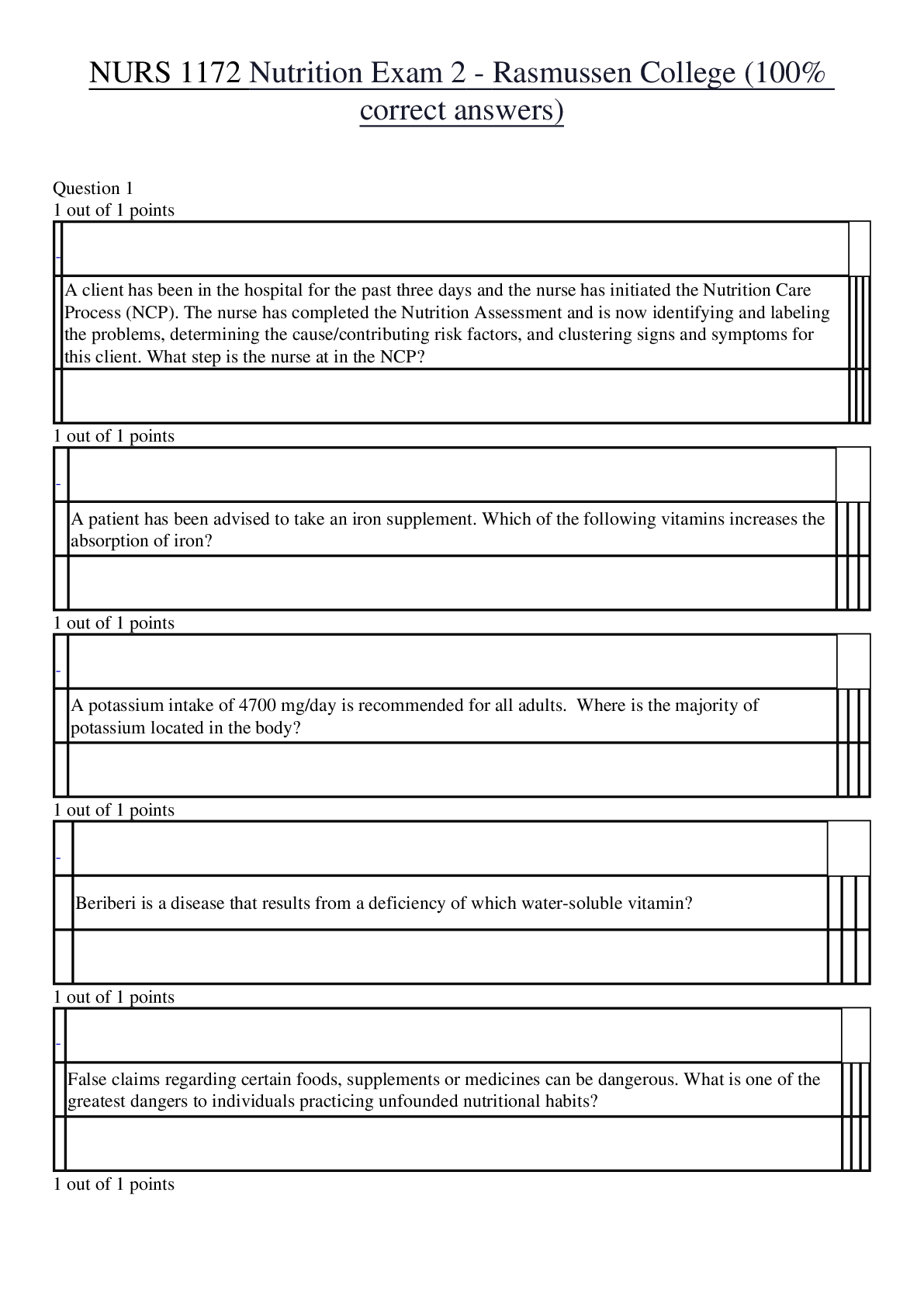
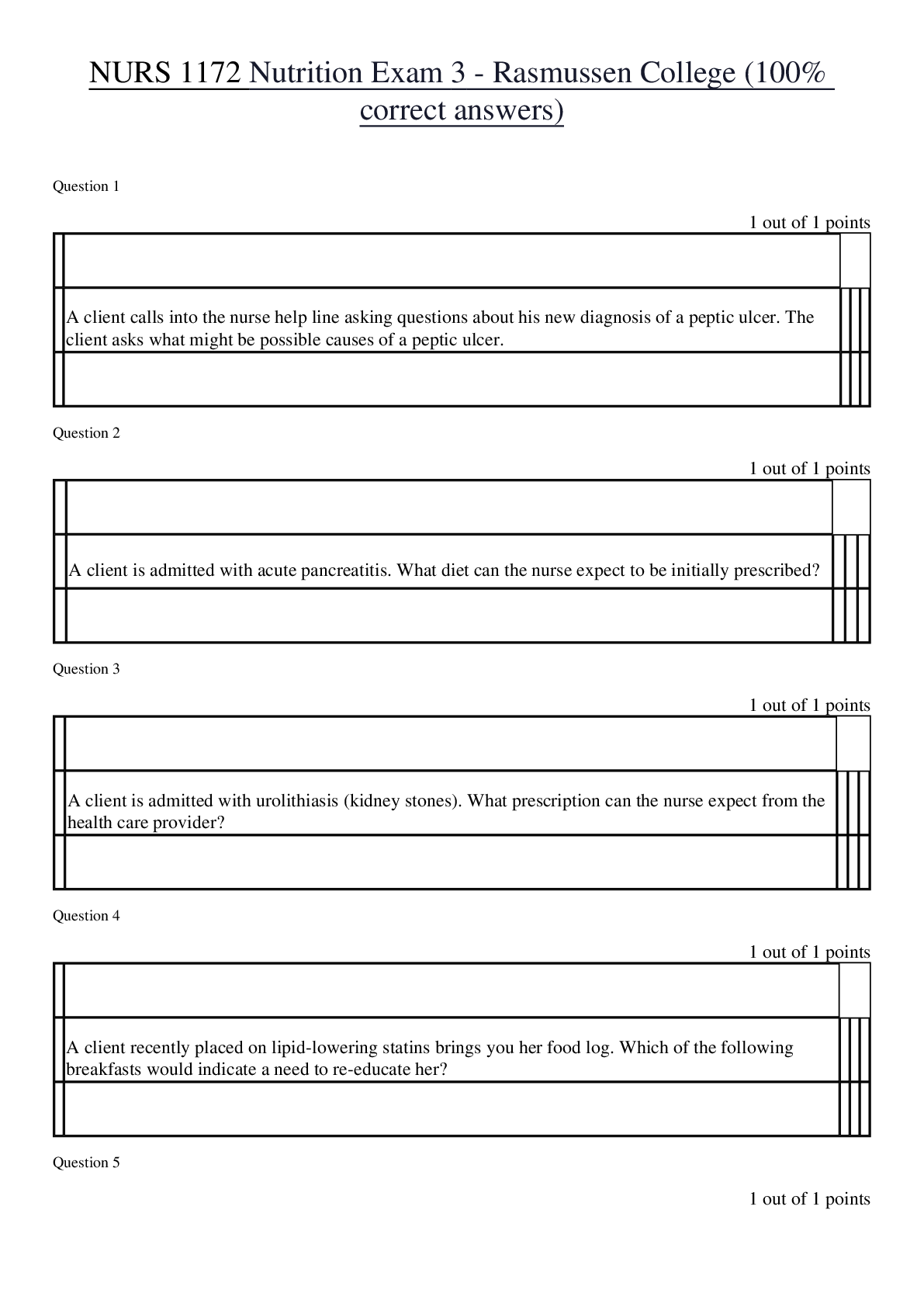
oxidation loss of electrons, positive charge atoms (anode) reduction gain of electrons, negative charged atoms (cathode) forms of corrosion uniform, crevice, galvanic (dissimilar me... tals) if two metals have to be couple then best to couple metals close together in the galvanic series, pitting, intergranular, selective leaching (dezincification, graphitization), velocity phenomena, environmental cracking (corrosion fatigue, hydrogen embrittlement, SCC (Carbonates/bicarbonates) High PH Low hydrogen, alkaline, easy polarization Low PH high hydrogen, acidic, harder to polarize High oxygen/High Hydrogen concentration harder to polarize, work at the cathode only, called cathodic depolarizers. factors affecting corrosion A. anything that affects polarization. (temp. etc...) B. concentration cells (oxygen, temp, etc.) In an oxygen concentration cell, what is the anode? (i.e. the area with more or less oxygen?) In a metal ion cell, which area is the anode (i.e. the area with the greater of lower concentration)? C. Anaerobic bacteria (MIC) Absence of oxygen, depolarizer polarization a. corrosion current reduces with polarization b. polarization increases with CP current applied c. Occurs at surface of both anode and cathode. shielding a. shorted casings b. proximity of other structures c. reinforced concrete amphoteric materials corrode at low and high pH on potential native + polarization + IR drop polarized potential (off) native + polarization kirchoff's laws [Show More]
Last updated: 2 years ago
Preview 1 out of 23 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$10.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Nov 29, 2022
Number of pages
23
Written in
Additional information
This document has been written for:
Uploaded
Nov 29, 2022
Downloads
0
Views
57