CHEM 104 Experiment 4: Molarity of Vitamin C - Portage Learning
Document Content and Description Below
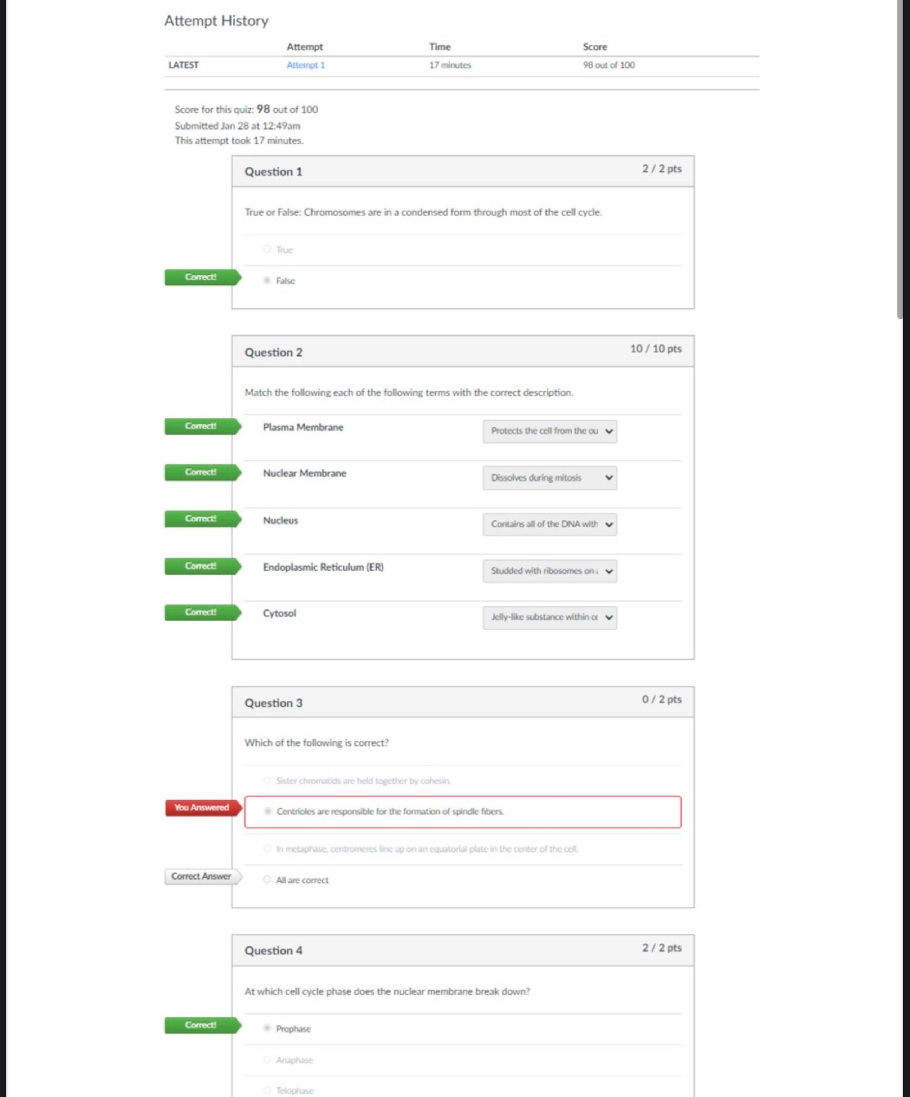
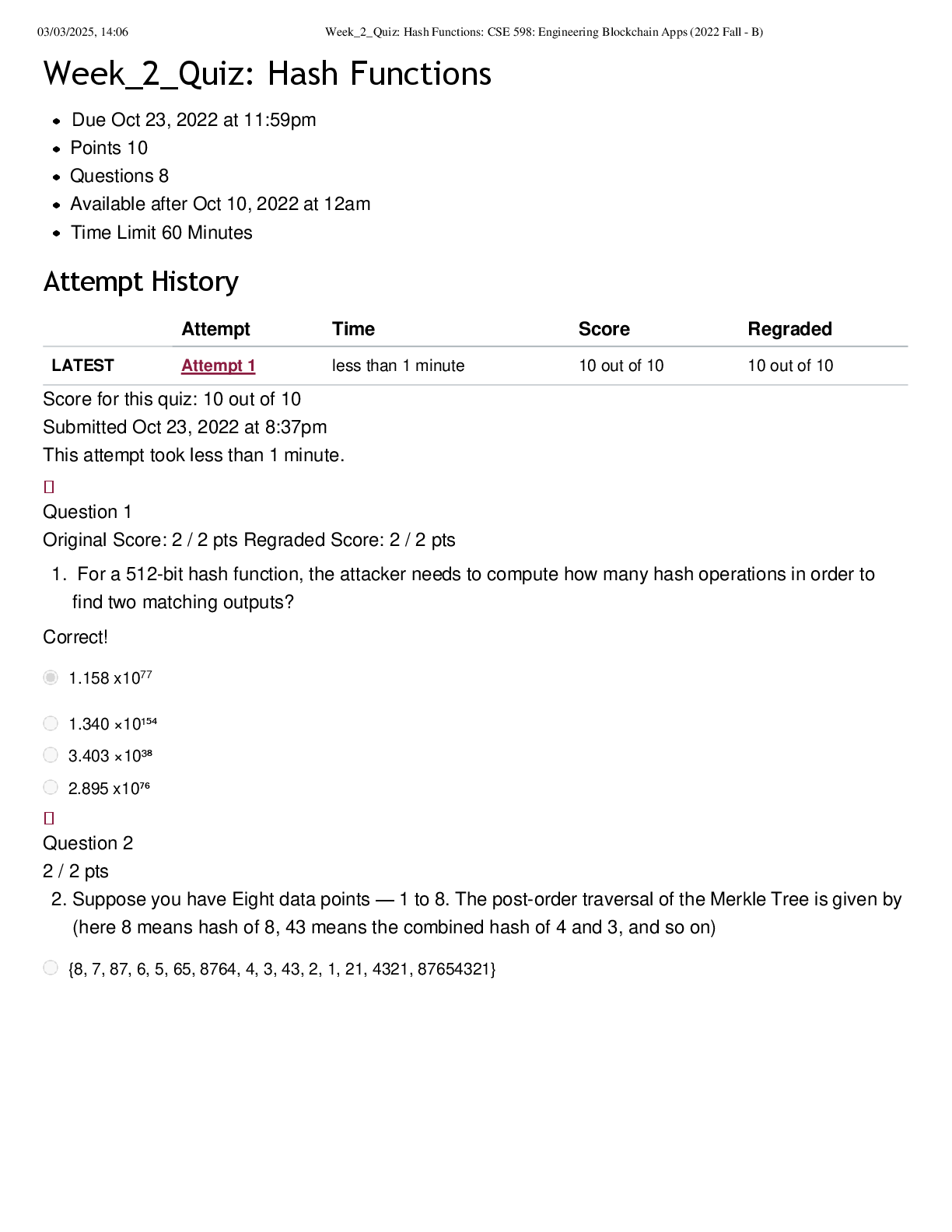
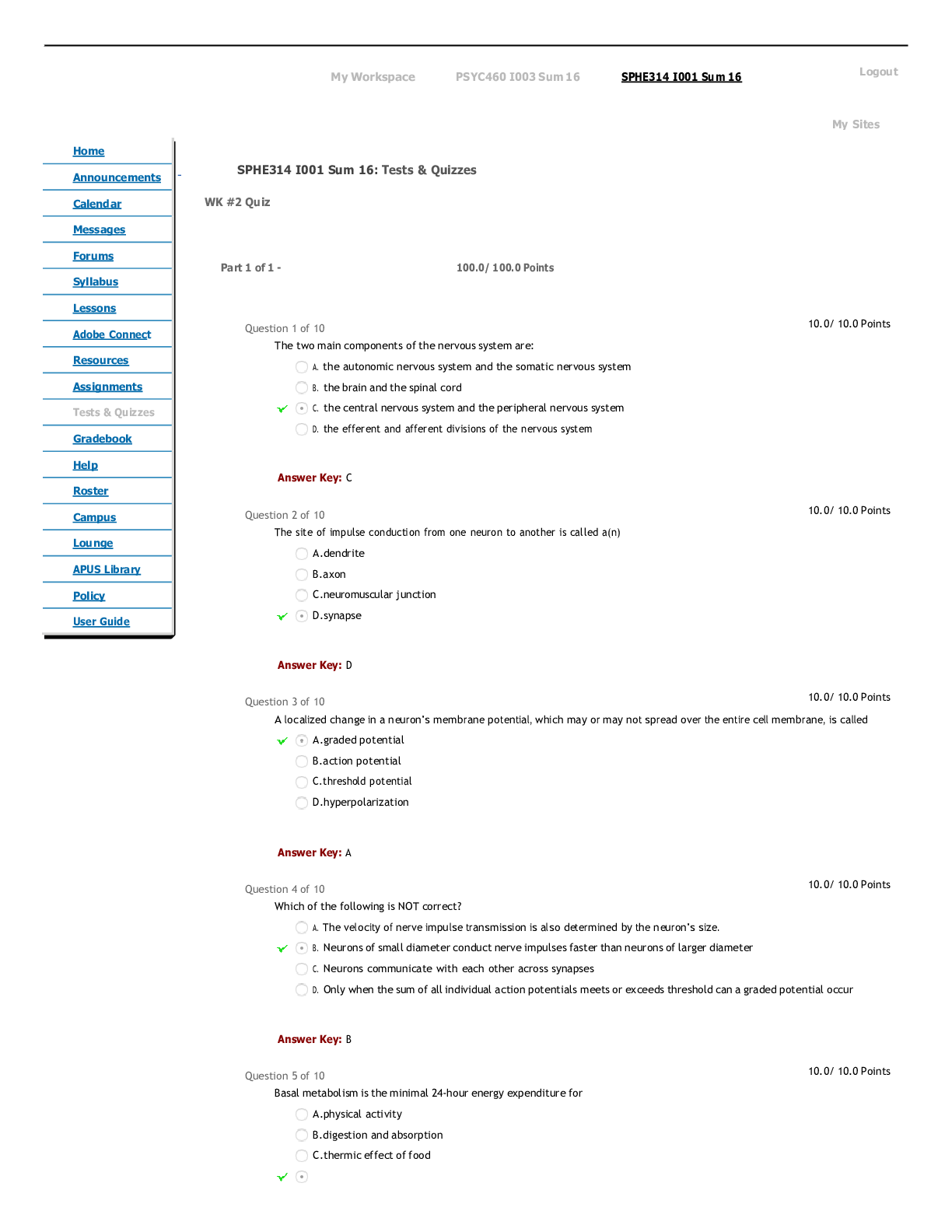
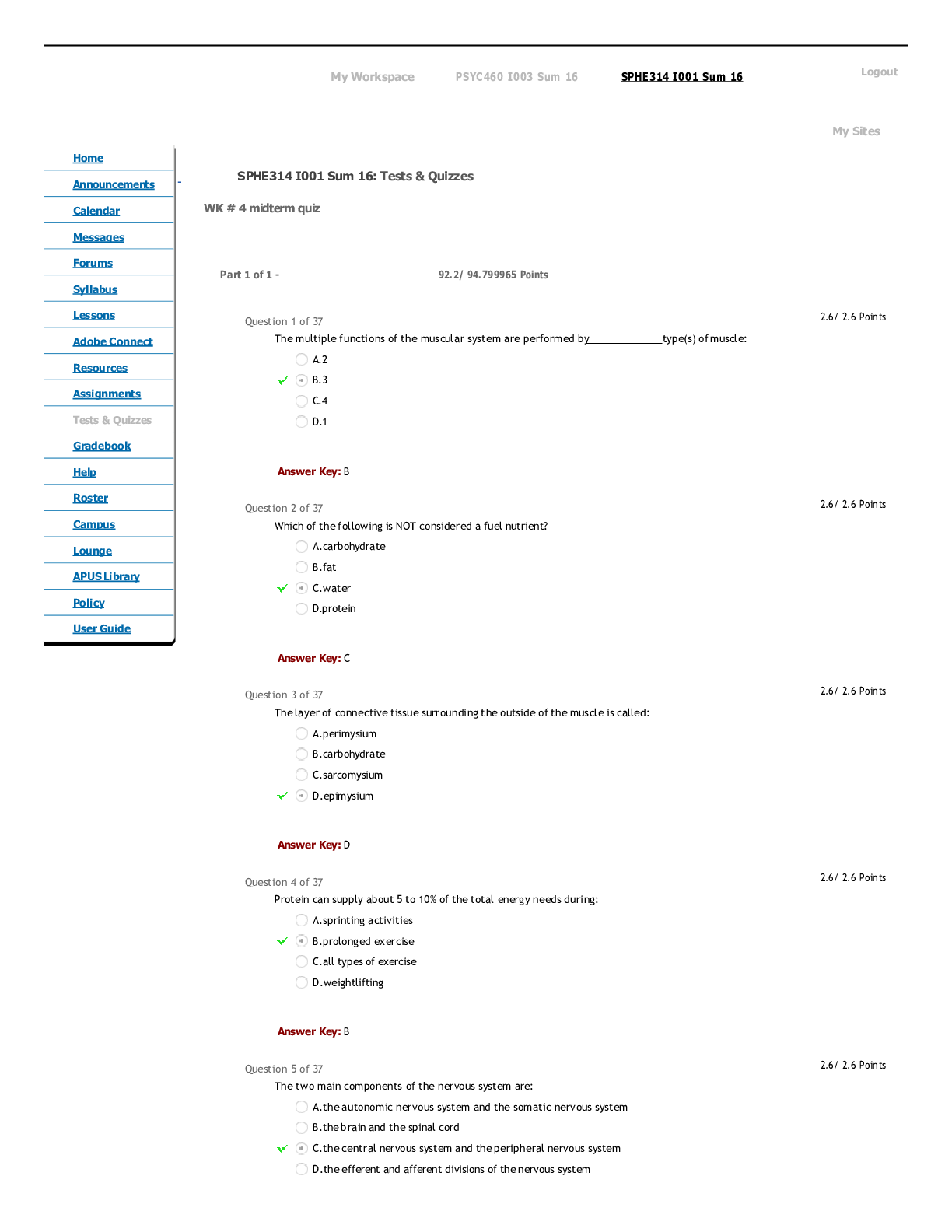
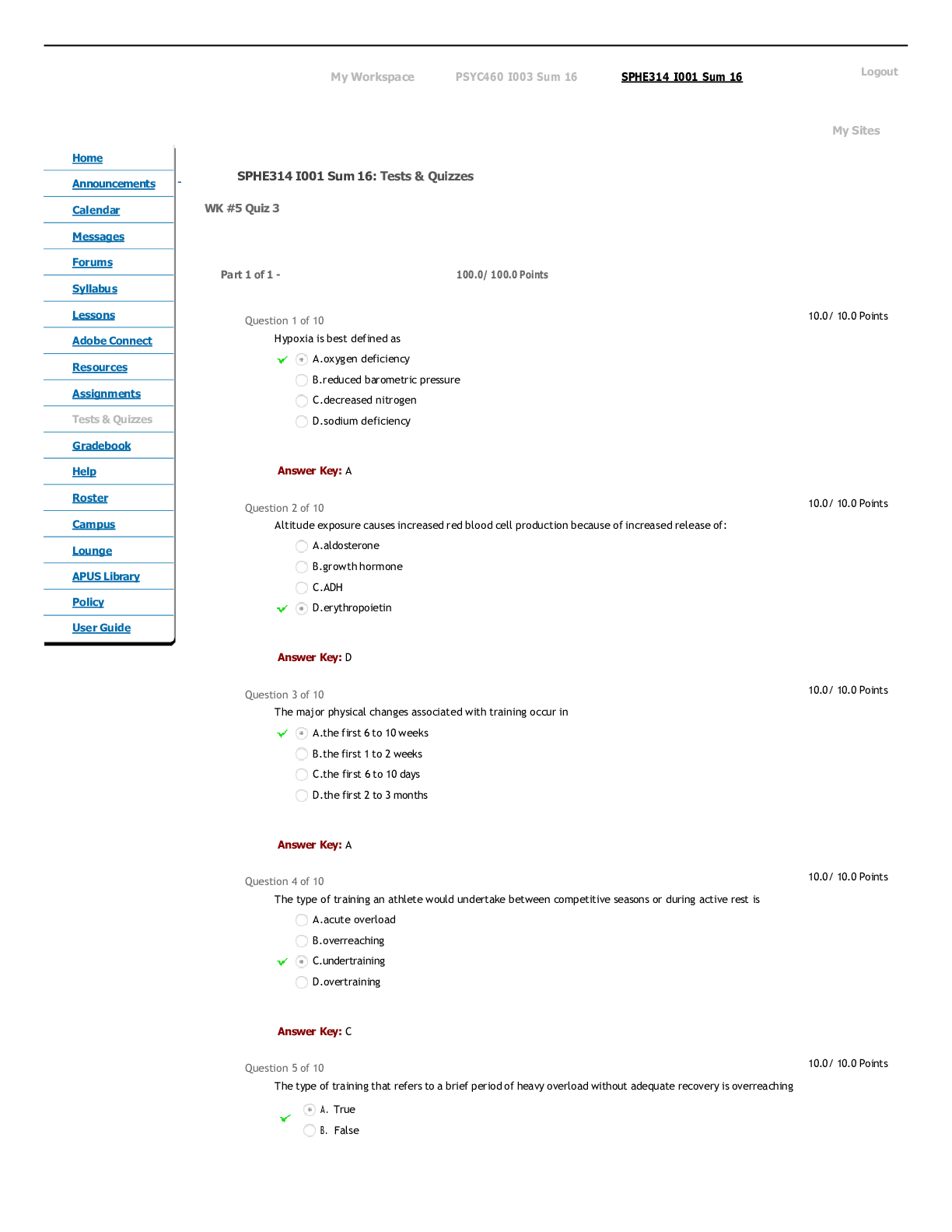
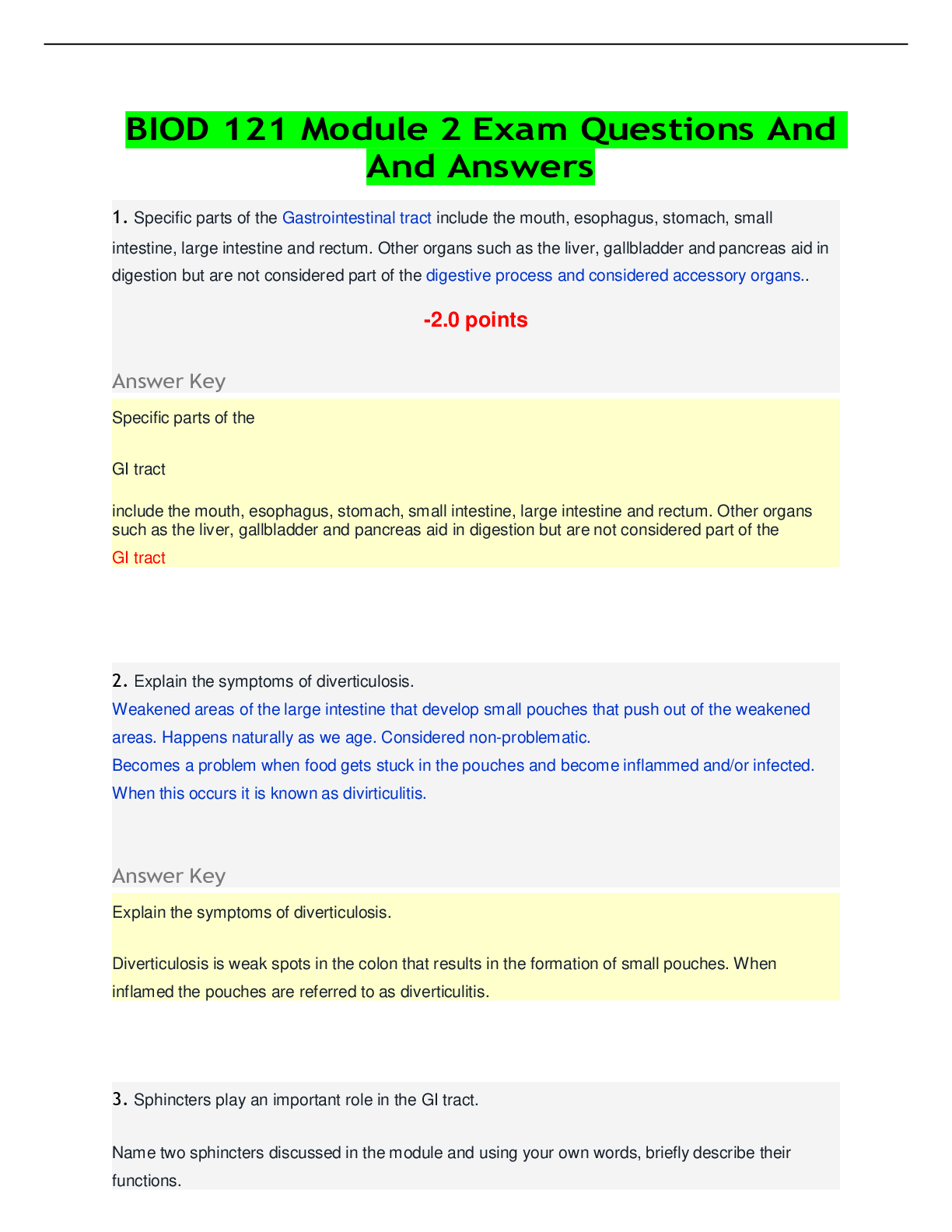
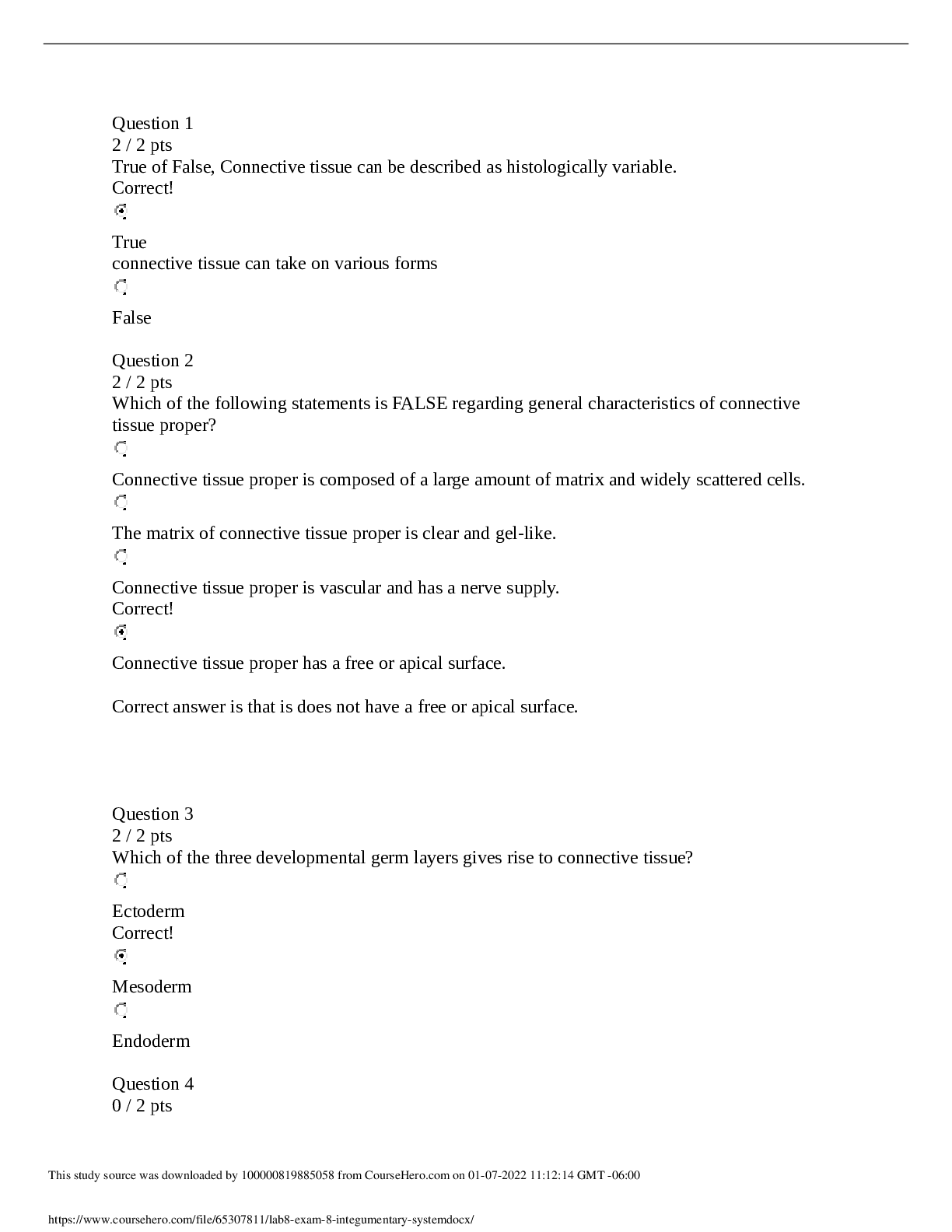
CHEM 104 Experiment 4: Molarity of Vitamin C - Portage Learning Purpose: The purpose of this lab is to determine a better understand of solutions and concentrations relating to vitamins, specifically... vitamin C or ascorbic acid. In order to do so, we measured the vitamin C in a few different fruits to determine the concertation of vitamin c in each. Procedure: Part 1: Determining the vitamin c concentration of iodine 1. Iodine was placed in a buret. 2. A solution was made from the commercial vitamin C by weighing out 0.465 g on a balance scale, ensuring to tare (zero) the balance after added the weigh paper. 3. The vitamin C was them placed in a volumetric flask & 250 mL of H2O was added, swirling to mix & ensure that it is all dissolved. 4. Molarity of vitamin c was calculated 5. Next, we poured a 10 mL solution of 0.1 mol vitamin C into a graduated cylinder. 6. 2-3 drops of acetic acid and starch were added. 7. We anticipate the endpoint color to be purple 8. Iodine was slowly added from the buret, remember to swirl occasionally. 9. This was continued until the purple colored solution was established 10. Moles of iodine = moles of vitamin c titrated 11. The concentration of iodine was then calculated Part 2: Determining the vitamin c concentration in orange juice 1. Prepare 20 mL of orange juice & place into a graduated cylinder 2. 30 mL of orange juice was added to make a total of a 50 mL solution, in doing so the solution is more dilute but it is more visible so we can determine the endpoint more accurately 3. 2-3 drops of acetic acid and starch were added 4. Iodine was slowly added from the buret, remember to swirl occasionally. [Show More]
Last updated: 1 year ago
Preview 1 out of 3 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Also available in bundle (1)

CHEM 104 EXPERIMENT 1-8 BUNDLE - PORTAGE LEARNING
CHEM 104 EXPERIMENT 1-8 BUNDLE - PORTAGE LEARNING
By Nurse Henny 1 year ago
$50
8
Reviews( 0 )
$13.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Aug 25, 2023
Number of pages
3
Written in
Additional information
This document has been written for:
Uploaded
Aug 25, 2023
Downloads
0
Views
174