Part 1: Complete the Labster Lab: Solution Preparation: From salt to solution
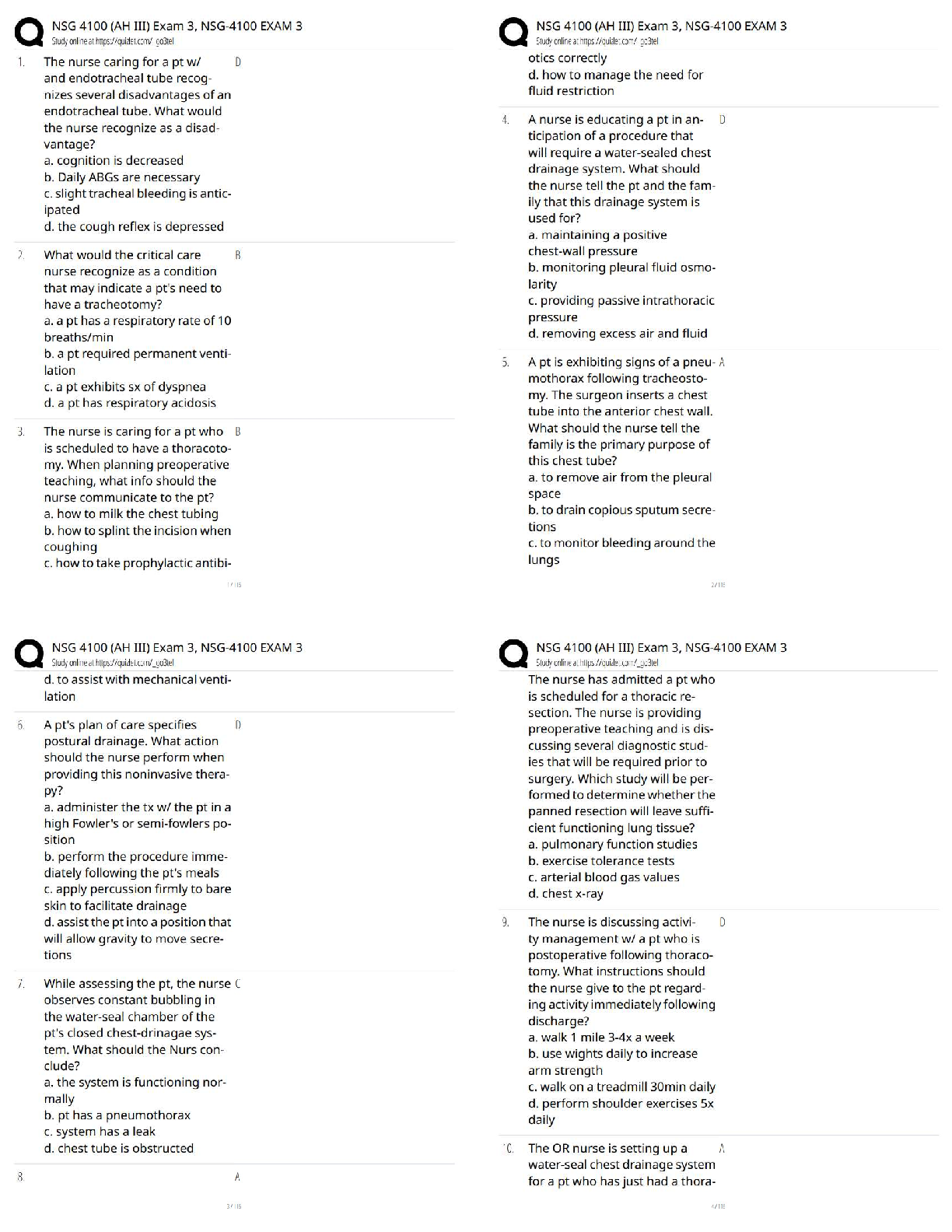
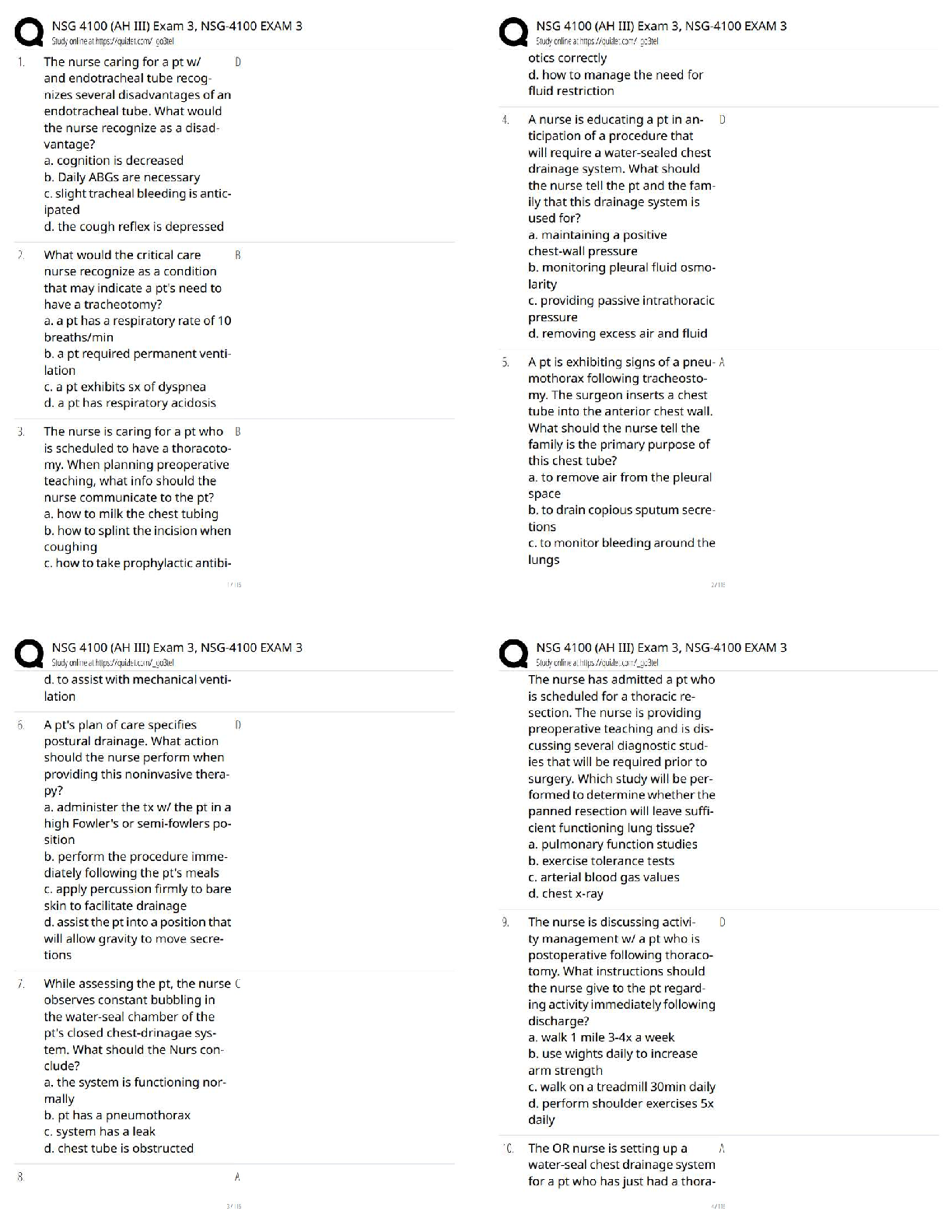
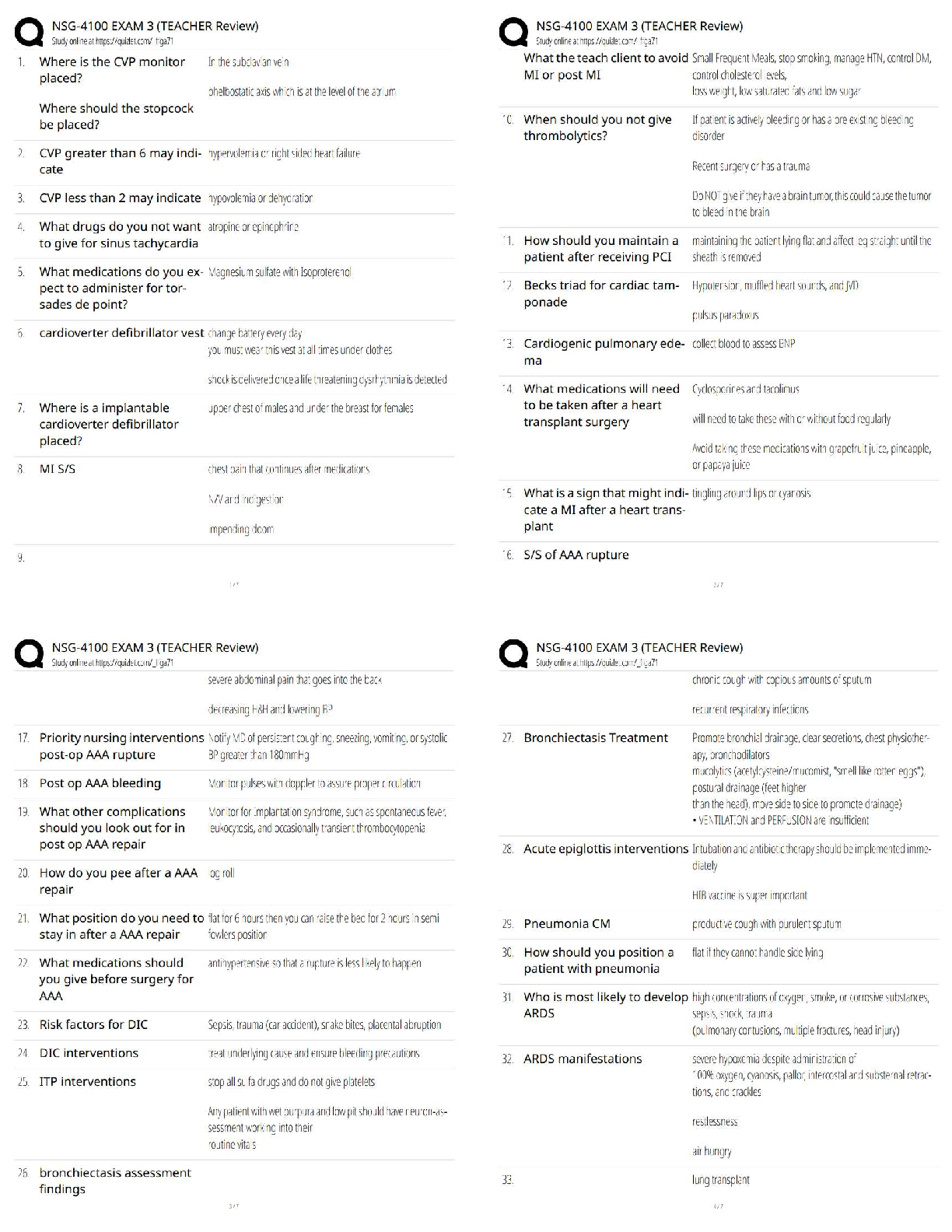
1. Which equation do we need to find out how many moles of NH4Cl would there be in
500 ml of a .300 M solution of it? n= CxV
2. How do we t
...
Part 1: Complete the Labster Lab: Solution Preparation: From salt to solution
1. Which equation do we need to find out how many moles of NH4Cl would there be in
500 ml of a .300 M solution of it? n= CxV
2. How do we then get from moles (n) to mass (m)? m=nxM
3. What was the first step of using the balance before adding anything to a weighing dish?
Tare the balance with the weighing dish and the balance closed
4. Why is it that the volumetric flask is the right choice here? It’s ideal for mixing different
substances in. It has the precision we need.
5. How sure would you say about the concentration at this point? It’s inaccurate.
6. What do you do if you add a bit too much solvent when filling the volumetric flask to the
mark? Start over, solution cannot be saved.
Part 2: Report and Reflection
Purpose: Describe in complete sentences and in your own words, the purpose of this
experiment.
This experiment shows how to conduct experiments using a solute and a solvent. It shows what
to do to make sure everything is accurate, so the experiment is not tainted. The experiment also
shows the proper equipment to use.
Observations: Record three observations from the simulation.
1. You must tare anything that is used to weight a substance beforehand.
2. A volumetric flask is the best option for mixing a solute and a solvent.
3. Sometimes you need to start an experiment over due to a small miscalculation or too
much of a solute or solvent which is okay.
Answer the questions below:
1. In this lab, you learned how to prepare solutions with a precise concentration.
Where in your nursing career might this skill be needed?
There are going to be times as a nurse that a solute and a solvent are going to
need to be mixed if they are not already. Knowing how to mix properly will help
be able to mix a solute and a solvent when needed.
2. Which piece of glassware did you use to prepare your solution in this lab and
why?
I used a volumetric flask because it is precise, and it helps mix the solute and
solvent.
This study source was downloaded by 100000796615030 from CourseHero.com on 08-05-2021 14:54:32 GMT -05:00
https://www.coursehero.com/file/63578726/OL-Lab-6-Solution-Preparation-From-salt-to-solutiondocx/
This study resource was
shared via CourseHero.com
CHEM120 OL, Week 3 Lab
3. Calculate how many grams of NaCl are required to make 100 mL of a 1 M NaCl
solution. Show your work in the space below.
1 mole = 63.068g NaCl (Added 22.99 + 40.078)
63.068 g NaCl/100 mL=.63068 g NaCl
4. Reflection: Consider what you learned from this simulation. Reflect on three to
four key concepts that you learned in this lab exercise. How could the lessons
learned in this virtual lab relate to a real world situation in the community/world or
your future career? Be specific in your answer (this should require 5-10
sentences).
A chemical can be unknown in an experiment which is why an experiment is
being conducted. An experiment can tell what kind of chemical is present, the
weight, and how many moles is present. This experiment showed just that to
determine how to decipher what chemical was present since bottle’s label was
worn. There are times that scientists need to do this because the bottles are
worn so they need to conduct experiments to determine what it is.
Grading Rubric:
Activity Deliverable Points
Part I Complete simulation and answer all questions 8
Part II Complete lab report and answer questions
Observation (3 points)
Purpose (1 point)
Questions (3 points)
Reflection (2 points)
9
Total Complete all lab activities 17
[Show More]