LIFE THE SCIENCE OF BIOLOGY 11TH EDITION SADAVA TEST BANK
Document Content and Description Below
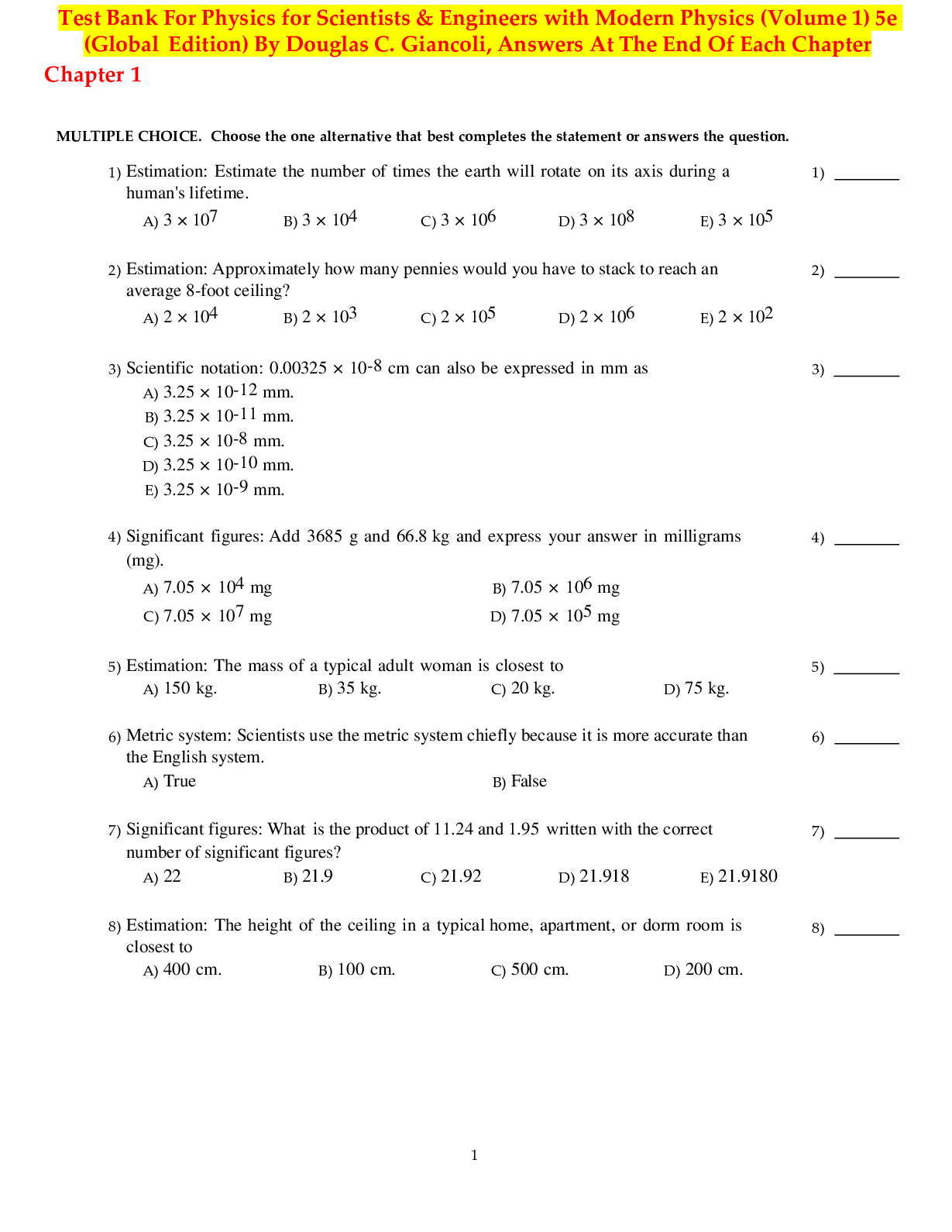
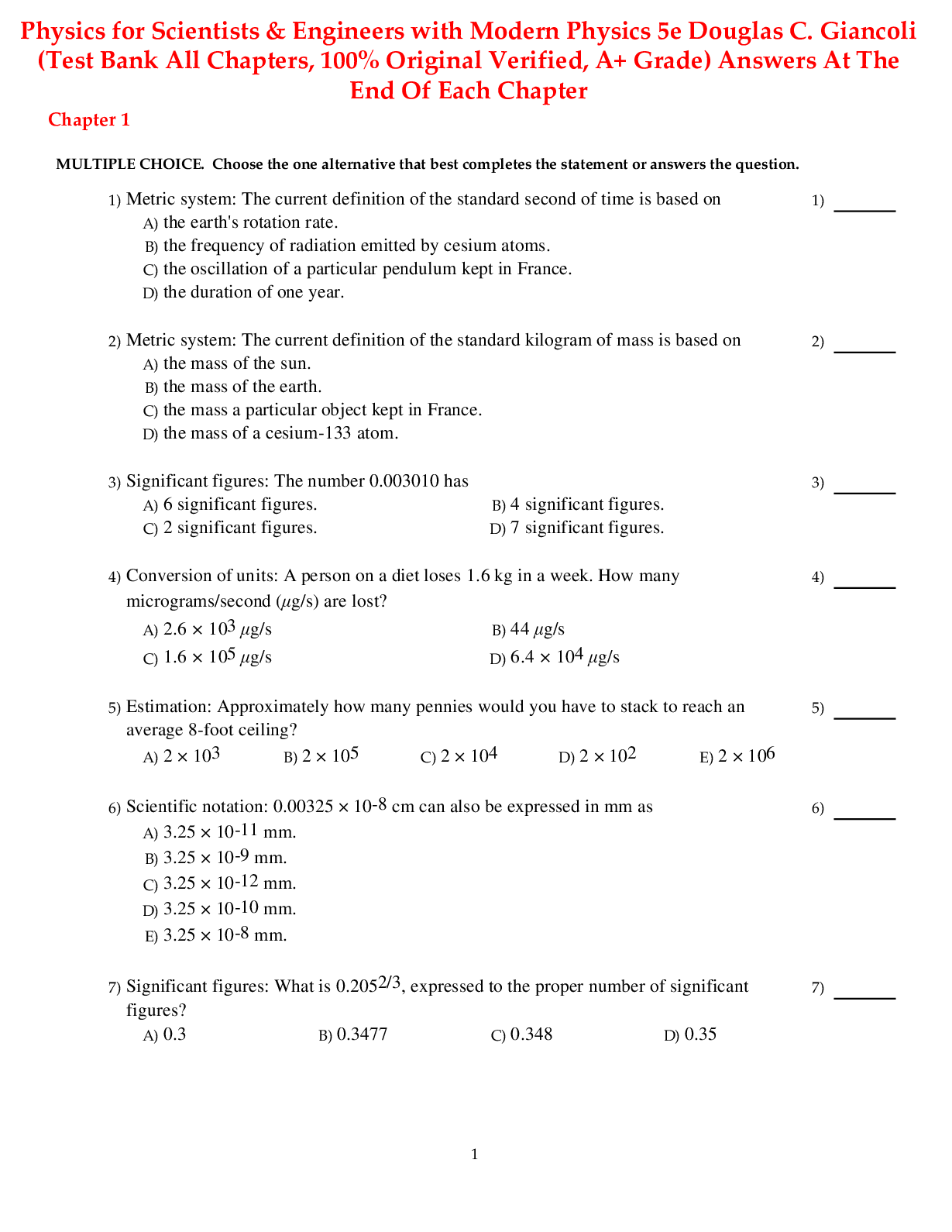
LIFE THE SCIENCE OF BIOLOGY 11TH EDITION SADAVA TEST BANK-1. An atom with has an atomic mass of 14. a. 14 neutrons b. 14 electrons c. 7 neutrons and 7 electrons d. 7 protons and 7 electrons e. 6 ... protons and 8 neutrons Answer: e Learning Outcome: 2.1.1.a Describe the structure of an atom. Bloom’s Level: 3. Applying 2. Which statement about an atom is true? a. Only protons contribute significantly to the atom’s mass. b. Only neutrons contribute significantly to the atom’s mass. c. Only electrons contribute significantly to the atom’s mass. d. Both protons and neutrons together contribute significantly to the atom’s mass. e. Both protons and electrons together contribute significantly to the atom’s mass. Answer: d Learning Outcome: 2.1.1.a Describe the structure of an atom. Bloom’s Level: 1. Remembering 3. What is the difference between an atom and an element? a. An atom is made of protons, electrons, and (most of the time) neutrons; an element is composed of only one kind of atom. b. An element is made of protons, electrons, and (most of the time) neutrons; an atom is composed of only one kind of element. c. An atom does not contain electrons, whereas an element does. d. An atom contains protons and electrons, whereas an element contains protons, electrons, and neutrons. e. All atoms are the same, whereas elements differ in structure and properties. Answer: a 4. In the history of the discovery of the parts of an atom, the neutron was discovered after the proton and electron. What property of a neutron made it more difficult than the proton or electron to discover? a. Diameter b. Location in the nucleus c. Mass d. Lack of charge e. Presence in isotopes Answer: d Learning Outcome: 2.1.2.a Compare and contrast the properties of protons, neutrons, and electrons. Bloom’s Level: 3. Applying 5. The number of protons in a neutral atom equals the number of a. electrons. b. neutrons. c. electrons plus neutrons. d. neutrons minus electrons. e. isotopes. Answer: a Learning Outcome: 2.1.3.a Explain why atoms typically have no overall electrical charge. Bloom’s Level: 1. Remembering 6. Which of the following statements about the atom is true? a. There are usually more protons than electrons in an atom because the negative charge of an electron is larger than the positive charge of a proton. b. The negative charge of an electron adds mass to an atom without influencing other properties. c. In an atom with a neutral charge, the number of electrons is equal to the number of protons. d. The number of electrons determines whether an atom of an element is radioactive. e. The energy level of electrons is higher in shells close to the nucleus of the atom. Answer: c Learning Outcome: 2.1.3.a Explain why atoms typically have no overall electrical charge. Bloom’s Level: 2. Understanding 7. A lithium atom contains three protons. For this atom to remain inert in an electric field, it must also contain a. three neutrons. b. three electrons. c. two neutrons and two electrons. d. no electrons. e. no neutrons. Answer: b Learning Outcome: 2.1.3.a Explain why atoms typically have no overall electrical charge. [Show More]
Last updated: 2 years ago
Preview 1 out of 90 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$18.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Aug 25, 2021
Number of pages
90
Written in
Additional information
This document has been written for:
Uploaded
Aug 25, 2021
Downloads
0
Views
105