BioChemistry > QUESTIONS & ANSWERS > BIOL 3362 - Exam 3 Study Guide (Biochemistry, Fatty Acids). (All)
BIOL 3362 - Exam 3 Study Guide (Biochemistry, Fatty Acids).
Document Content and Description Below
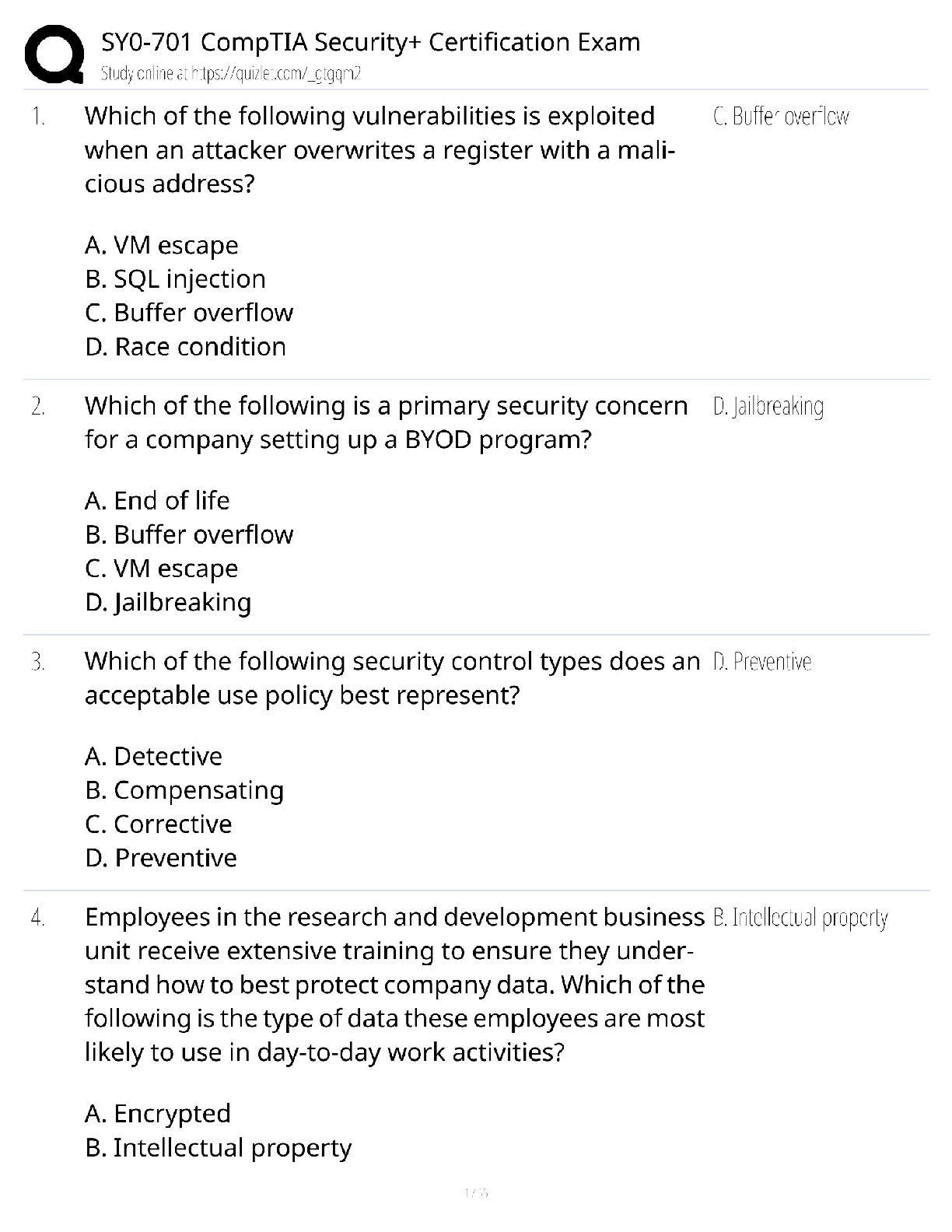
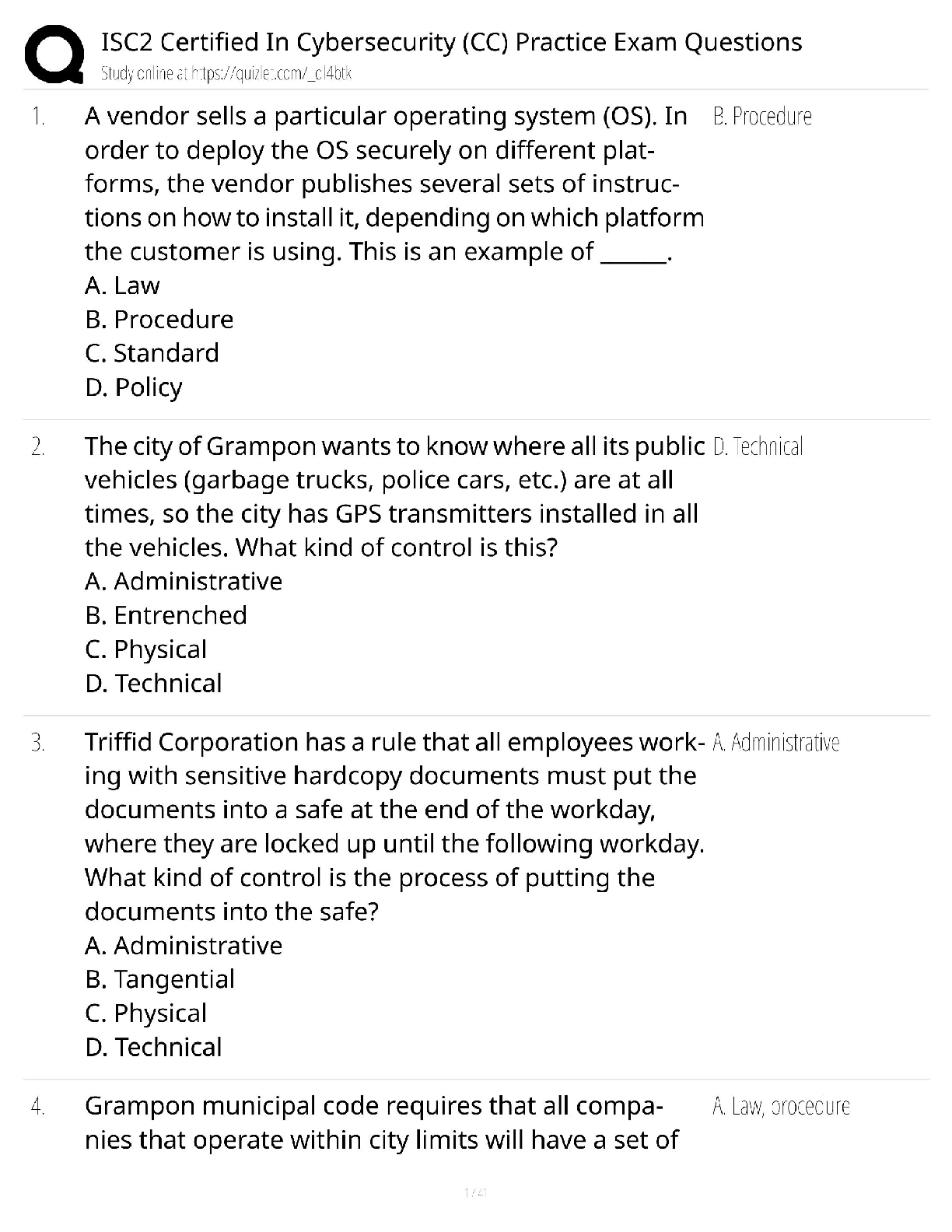
BIOL/CHEM 3362 - BIOCHEMISTRY II EXAM 3 STUDY GUIDE 1. How are the chiral carbons on the glycerol of the trigycerides determined? C1 is the carbon that would lead to S-configuration if its ... priority was increased. (chapter 8 slide 44) 2. How are the archaeal membrane lipids different from membrane lipids found in bacteria and eukaryotes? They are connected to the enantiomers of the bacteria and eukaryotes glycerol. (chapter 8 slide 49-50), Archaea vs bacteria:snG1P vs snG3P (enantiomers). Bacteria also have an abundance of unsaturated molecules compared to Archaea. Archaeal membranes are diether lipid isoprenoids and bacteria are diester lipids. 3. How do short, long, saturated and unsaturated fatty acids affect solidity or liquidity of fats and oils? More saturated fats tend to have a higher melting point and are often solid at room temperature, while unsaturated fats have a lower melting point (Unsat=Unsolid). Trans fats have a trans double bond but are still considered as saturated fats. Trans fats are not recognized by the human metabolism. Saturated vs unsaturated contribution to melting point of chain length and cis-double bonds 1. As the molecular weight increases, the melting point increases. 2. Unsaturated fatty acids have lower melting points than the saturated fatty acids. Melting point: Lower melting point of unsaturated fatty acids - The reason for this phenomenon can be found by a careful consideration of molecular geometries. The tetrahedral bond angles on carbon results in a molecular geometry for saturated fatty acids that is relatively linear although with zigzags. This molecular structure allows many fatty acid molecules to be rather closely "stacked" together. As a result, close intermolecular interactions result in relatively high melting points. Cis-double bonds: On the other hand, the introduction of one or more double bonds in the hydrocarbon chain in unsaturated fatty acids results in one or more "bends" in the molecule. The geometry of the double bond is almost always a cis configuration in natural fatty acids. These molecules do not "stack" very well. The intermolecular interactions are much weaker than saturated molecules. As a result, the melting points are much lower for unsaturated fatty acid 4. What does “omega” designate in omega fatty acids? The term omega in fatty acids refers to the terminal carbon atom farthest from the functional carboxylic acid group (–COOH) (Chapter 8 slide 26). Defining an Omega 6 would be identified by the the 6th carbon from the Omega end, because it is the 1st C from the omega end that introduces a double bond. 5. Which desaturation enzymes are common to the processing of omega-3 and omega-6 fatty acids? (Δ-5 / Δ-6) desaturase, lipoxygenase (Ch. 8, slide 28-30) 6. Which bonds do phospholipases cleave on phosphoglycerides? Phospholipase A1 hydrolyzes the acyl group attached to the 1-position. Phospholipase A2 hydrolyzes the acyl group attached to the 2-position to form fatty acid and lysophospholipid products. (Phospholipase C-hydrolizes phosphate group on glycerol side, Phospholipase D on opposite side, not written but in figure) (Ch. 8 slide 78) 7. What are the products of saponification reaction? Alkali hydrolysis of triacylglycerols yields to formation of glycerol and free fatty acids (salt of free fatty acids - soap) as products. 8. Why triacylglycerols are not found in the cell membranes? They are nonpolar (not amphipathic) Triacylglycerols (formerly called triglycerides) are complex lipids. Triacylglycerols act as energy storage molecules, especially in adipose tissue; triacylglycerols are also found in lipoproteins. Triacylglycerols are not found in membranes, because they are essentially entirely non-polar. Triacylglycerols consist of three fatty acid molecules forming ester links to glycerol. A triacylglycerol molecule can be comprised of different fatty acids, or of three identical fatty acids. 9. Which tissue contains more gangliosides? -Gangliosides contain three or more sugar residues, with N-acetyl-neuraminic acid, a more complicated monosaccharide derivative, at the end of the chain. Gangliosides are important components of nerve cells -Nervous tissue -Gangliosides have antigenic properties (Ch. 9, slide 77) 10. Which lipids are linked to the pathogenicity of neurodegenerative diseases? Sphingolipid (Ch 8 slide 76) 11. How the fatty acids are denoted according to IUPAC nomenclature rules? Length of C-chain (Ch 8 slide 9) # carbons: # double bonds ... 18:2, cisΔ9,12 linoleic acid. Double bonds in fatty acids usually have the cis configuration. Each cis double bond causes a kink in the chain, or a bent tail. Most naturally occurring fatty acids have an even number of carbon atoms —12-24. 12. What factors influence the fluidity and phase transition temperature of a membrane? Fluidity Factors: Temp and Lipid Type. Long chain FA’s decrease membrane fluidity, Unsaturated (Unsat= unsolid=noncomaptacable cause of kinks or double bonds), Cholesterol because of its structure tends to decrease membrane fluidity. Temperatue affects membrane fluidity but it’s dependent on the FA compositon (Membranes rich in Phospholipids w/ unsaturated tails remains fluid at lower temps because kinks prevent the compaction) Higher Melting point for longer chains.) Membrane fluidity is the physical property that is associated with the lateral movement of lipids in the cell membrane. • Membrane fluidity depends on temperature and type of lipids: - long chain fatty acids decrease membrane fluidity - unsaturated fatty acids increase membrane fluidity - cholesterol, because of its structure tends to decrease membrane fluidity • Membrane fluidity has an effect on the movement of other chemical entities in the cell membrane, such as proteins 13. How is the Ras protein anchored to the cell membrane? Prenylation or isoprenylation (thioester-linked prenyl anchors): the addition of isoprene based farnesol (15-C) and geranylgeraniol (20-C) groups to a cysteine (dipthong) near the C terminus. It is always at a Cys residue. This is a Post-Translational Modification. (Human Ras protein plays an important role as a molecular switch in a signal transduction cascade responsible for cell proliferation, differentiation, and apoptosis.) 14. Which lipid enables curvature of lipid membrane more readily? Phosphatidylcholine, because it has no preferred curvature it predominates as a lipid membrane molecule used in curvature. phosphatidylcholine (PC) is cylindrical in shape and has no preferred curvature. Therefore, phosphatidylcholine has no micellar preference and would generally assemble into lamella and is the main component of bilayer membranes 15. Why facilitated transport across membrane is a saturable process? Saturable because substrates can overwhelm the channel 16. How do small hydrophobic molecules cross a membrane? Forms solvation shell. A small molecule might traverse a lipid bilayer membrane in the following way: first, it sheds its solvation shell of water; then, it becomes dissolved in the hydrocarbon core of the membrane; finally, it diffuses through this core to the other side of the membrane, where it becomes resolvated by water. 17. Which lipids form/maintain the liquid-ordered raft structures in the cell membrane? Microdomains are postulated to be aggregates of specific proteins, cholesterol, and Sphingolipids (glycosphingolipids) with long, saturated fatty acyl chains (particularly ceramides and gangliosides), and they are termed membrane rafts, an apt description because these protein-rich lipid aggregates move together laterally in the “sea” of phospholipid. (page 281, 5th ed, Biochemistry) 18. How does the pancreatic lipase cleave triglycerides? Pancreatic Lipase cleaves from C1 to C3 position, leaving other lipases and esterases to attack the C2 position. Lipase is a hydrolysis reaction on the acyl group. 19. How do isoprene blocks form long chains? Mainly Head-Tail linkage but it can do tail-tail linkage 20. Which amino acid is used in lipid anchoring of proteins via prenylation? Cysteine 21. What is oubain? Diets rich in sodium also enhance the release of adrenal endogenous ouabain-like compounds, which inhibit Na+,K+-ATPase activity, resulting in increased intracellular Na+ and Ca2+ concentrations in vascular smooth muscle cells, thus increasing the vascular tone, with a corresponding increase in blood pressure. Ouabain is a Na+,K+-ATPase inhibitor compound. It can be found in the roots, stems, leaves, and seeds of certain plants native to eastern Africa. • It binds to the pump and ceases its function, leading to an increase of intracellular sodium. • Diets rich in sodium also enhance the release of adrenal endogenous ouabain-like compounds, which inhibit Na+,K+-ATPase activity, resulting in increased intracellular Na+ and Ca2+ concentrations in vascular smooth muscle cells, thus increasing the vascular tone, with a corresponding increase in blood pressure. • Persistent increase in vascular tone is characteristic of chronic hypertension. Sodium-rich diets promote hypertension Ouabain is classified as a cardiac glycoside - in lower doses, it can be used medically to treat hypotension and some arrhythmias. is a Na+,K+-ATPase inhibitor Glycoside used to treat Hypotension and some arrhythmias 22. Why water transport across cell membrane requires a protein channel? 28.What are the factors determining the molecular selectivity of aquaporin for water? Because water only moves by osmosis, in order to direct the movement of water, cells in the kidney set up strong osmotic gradients using Na+. By regulating these gradients and specific channel proteins, kidney cells exert precise control over loss or retention of water and electrolytes Aquaporins constitute a family channel proteins, which are highly selective for water- reject both solutes and proton flow. AQP1 from human red cells is the first aquaporin for which a 3D structure has been published. An open aquaporin transport channel facilitates transfer of 3 billion water molecules per second. Aquaporins consist of six membrane-spanning segments arranged in two hemipores, which fold together to form the channel. • The highly conserved NPA motifs form a size-exclusion pore, giving the channel its high sensitivity. • H2O molecules become aligned by interaction with Asn residues at the constriction site of aquoporin channel. Increases volume in order to provide water for all the needs of the cell. Transport w/o the aquaporin is possible but the cell would lose functionality. 23. What is the process/reaction kinetics of facilitated transport across membrane? Also an entropy-driven process, the transported species moves according to its thermodynamic potential with the help of an integral membrane protein (ion channel, etc.) Moves thing like passive transport but because some things are too large or polar, they 24. What is critical micelle concentration? The critical micelle concentration (CMC) of a surfactant is the concentration at which surfactant micelles form. At concentrations below the CMC of a surfactant, surfactant molecules may bind to the hydrophobic portion of an integral membrane protein, making the protein soluble in aqueous solution, but the protein will not be dissolved in surfactant micelles. Micelles only form above critical micelle temperature. CMC is the concentration of surfactants above which micelles form and all additional surfactants added to the system go to micelles. Once surface tension reaches a certain point, all additional molecules will go to micelles since the tension is at its limit. 25. Which cellular processes depend on lipid raft clustering on membranes? Signaling (B-cell receptors) 26. What are the key structural features of plasmalogens? A plasmalogen is a variant of an ether lipid in which the fatty alcohol has a double bond between carbons 1 and 2 (alkyl moiety is unsaturated). Plasmalogens can be classified as antioxidants since they terminate lipid-oxidation processes. The plasmalogens are easily react with a variety of free radicals in and around the lipid membrane, preventing the oxidation of highly polyunsaturated fatty acids. Products of plasmalogen degradation do not propagate lipid oxidation. Although cells rapidly incorporate and synthesize plasmalogens de novo, their plasmalogen contents can be deliberately increased by supplementation with precursors. 27. What is a hydropathy plot? How do we read the plot? A hydropathy plot reveals the topology of a transmembrane helical protein based on its amino acid sequence. If a measure of hydrophobicity is assigned to each AA, then the overall hydrophobicity of a segment of a polypeptide chain can be estimated. The hydropathy index for any segment is an average of the hydrophobicity values of its residues. 29. What are the differences between brown fat cells and white fat cells? White - store energy as triglycerides barely sufficient ring of cytoplasm surrounding a single lipid droplet nuclei are flattened and located in the periphery of the cell Brown - burn lipids for thermogenesis considerable volume of cytoplasm and contain multiple lipid droplets of varying size lots of mitochondria, heat generation nuclei are round and centrally located 30. What is role of carnitine in fatty acid metabolism? (carnitine is to transport long chain FA into the inner mitochondrial membrane) Carnitine carries fatty acyl groups across the inner mitochondrial membrane The process involves the coordinated actions of carnitine acyltransferases on both sides of the membrane and of a translocase that shuttles O-acylcarnitines across the membrane. • Acyl-CoA esters are formed inside the inner membrane in this way • Short chain fatty acids are carried directly into the mitochondrial matrix • Long-chain fatty acids cannot be directly transported into the matrix • Long-chain FAs are converted to acyl carnitines and are then transported in the cell 31. What is/are the use(s) of alpha-, beta-, and omega-oxidation of fatty acids? Alpha oxidation : Branched chain FAs with branches at odd-number carbons cannot be processed by β- oxidation. • α-oxidation is used to brake down branched fatty acids by removal of a single carbon from the carboxyl end. In humans, alpha-oxidation is used in peroxisomes to break down dietary phytanic acid, which cannot undergo beta-oxidation due to its β- methyl branch. • Phytanic acid α-oxidase decarboxylates with oxidation at the alpha position • β-oxidation occurs past the branch • The product of the phytanic acid oxidase, pristanic acid, is a suitable substrate for normal β-oxidation. Isobutyryl-CoA and propionyl-CoA can both be converted to succinyl-CoA, which can enter the TCA cycle. Beta oxidation: The oxidative breakdown of phenyl fatty acids observed by Franz Knoop. He observed that fatty acid analogs with even numbers of carbon atoms yielded phenyl acetate, whereas compounds with odd numbers of carbon atoms produced only benzoate is termed β-oxidation since it occurs through the sequential removal of 2-carbon units by oxidation at the β-carbon position of the fatty acyl-CoA molecule Omega oxidation : involve the oxidation the carbon most distance from the carboxyl. Omega oxidation is a process of fatty acid metabolism in some species of animals. It is an alternative pathway to beta oxidation that, instead of involving the β carbon, involves the oxidation of the ω carbon (the carbon most distant from the carboxyl group of the fatty acid). It occurs in the ER of liver and kidney cells. 32. Why is there fatty acid oxidation in peroxisomes? 33. What are the enzymes and reactions of beta-oxidation cycle? Acyl-CoA Dehydrogenase Oxidation of the C α -C β bond Mechanism involves proton abstraction, followed by double bond formation and hydride removal by FAD. Enoyl-CoA Hydratase: utilize a common catalytic strategy and add water across the double bond. Hydroxyacyl-CoA Dehydrogenase :Oxidizes the β -Hydroxyl Group Thiolase aka β -ketothiolase • Cysteine thiolate on enzyme attacks the β-carbonyl group • Thiol group of a new CoA attacks the shortened chain, forming a new, shorter acyl-CoA. • 34. What is the energy yield of fatty acid catabolism? How does ATP production yield change with fatty acid chain length?(SLIDE 56/ CHAPTER23) Repetition of the cycle yields a succession of acetate units • Thus, palmitic acid yields eight acetyl-CoAs • Complete β-oxidation of one palmitic acid yields 106 molecules of ATP • Large energy yield is consequence of the highly reduced state of the carbon in fatty acids • This makes fatty acids the fuel of choice many animals Complete β-Oxidation of One Palmitic Acid Yields 106 Molecules of ATP. Reduced coenzymes produced by β-oxidation and TCA cycle activity provide electrons that drive synthesis of ATP in oxidative phosphorylation. Complete oxidation of palmitoyl-CoA yields a net of 106 ATP. Pi(ATP) +ADP Pi(ATP) ATP AMP+PPi 35. How is propionic acid catabolized? What are the reactions used to convert and process propionate? Oxidation of Odd-Carbon Fatty Acids β-Oxidation yields propionyl-CoA • Odd-carbon fatty acids are metabolized normally, until the last three-C fragment - propionyl-CoA - is reached • Three reactions convert propionyl-CoA to succinylCoA • Note the involvement of biotin and B12 • Note the calculation of catalytic power of the epimerase reaction • Note pathway for net oxidation of succinyl-CoA The conversion of propionylCoA (formed from β-oxidation of odd-carbon fatty acids) to succinyl-CoA is carried out by a trio of enzymes. • Succinyl-CoA can enter the TCA cycle or be converted to acetylCoA. 36. Why is vitamin B12/cobalamin required for odd-number fatty acid catabolism? a vitamin B12 catalysed rearrangement which converts methylmalonyl CoA into succinyl CoA Fatty acids that contain double bonds or odd numbers of carbon atoms require ancillary steps to be degraded. • An isomerase and a reductase are required for the oxidation of unsaturated fatty acids, whereas propionyl CoA derived from chains with odd numbers of carbons requires a vitamin B12-dependent enzyme to be converted into succinyl CoA 37. Where does omega oxidation take place in the cell. the enzymes for ω oxidation are located in the endoplasmic reticulum of liver and kidney cells, instead of in the mitochondria as with β oxidation. 38. How and where are the ketone body formed? The liver acts as the body’s glucose reservoir, and helps to keep your circulating blood sugar levels and other body fuels steady and constant. The liver both stores and manufactures glucose depending upon the body’s need. The need to store or release glucose is primarily signaled by the hormones insulin and glucagon. During a meal, liver store sugar, or glucose, as glycogen for a later time when your body needs it. The high levels of insulin and suppressed levels of glucagon during a meal promote the storage of glucose as glycogen. When not eating – especially overnight or between meals, the body has to make its own sugar. The liver supplies sugar or glucose by turning glycogen into glucose by glycogenolysis. The liver also can manufacture necessary sugar or glucose by harvesting amino acids, waste products and fat byproducts by gluconeogenesis. When the body’s glycogen storage is running low, the body starts to conserve the sugar supplies for the organs that always require sugar. These include: the brain, red blood cells and parts of the kidney. To supplement the limited sugar supply, the liver makes alternative fuels called ketone bodies from fats. This process is called ketogenesis. The hormone signal for ketogenesis to begin is a low level of insulin. Ketones are burned as fuel by muscle and other body organs. And the sugar is saved for the organs that need it During high rates of fatty acid oxidation, primarily in the liver mitochondria, large amounts of acetyl-CoA are generated. • These exceed the capacity of the TCA cycle, and one result is the synthesis of ketone bodies, or ketogenesis. • The ketone bodies are acetone, acetoacetate and β -hydroxybutyrate • Source of fuel for brain, heart and muscle • Major energy source for brain during starvation. beta Hyroxybutyrate -------- ----------Acetyl CoA (provide metabolic energy )later feed to the Creb cycle 39. Why oxidation of unsaturated fatty acids yields less energy than oxidation of saturated fatty acids? How does the beta-oxidation of unsaturated fatty acids differ? How do energy yields differ? requires an extra enzyme to convert cis-double bond to trans - enoyl-coA isomerase - this allows for normal beta-oxdiation and conversion to acetyl-coa - oxidation of unsaturated fatty acids yields less energy than saturated fatty acids (so if you need energy, eat saturated fatty acids) - this is because first step is missing, so you lack FADH2 40. How does thiolase activity differ in ketone body synthesis as compared to beta- oxidation of fatty acids? Separate pathway ,enzyme invole in thermodynamic condition The accepted name for thiolase I is acetyl-CoA C-acyltransferase, while for thiolase II is acetyl-CoA C-acetyltransferase. • Thiolase I (3-ketoacyl CoA thiolase), which exhibits broad substrate specificity, is also called the degradative thiolase as it mainly catalyzes the final step in the β-oxidation pathway, shortening an acyl-CoA by the two-C acetyl CoA, the other product of the reaction. • Thiolase II (acetoacetyl CoA thiolase) uses only acetoacetyl Co A and is also called the synthetic thiolase as it catalyzes the condensation of two acetyl CoA to form the 4-C acetoacetyl CoA, an important starting molecule of hormone and cholesterol synthesi 41. How are the mixed micelles formed? 42. What is non-shivering thermogenesis? How does it occur? As in white fat, sympathetic stimulation promotes hydrolysis of triglyceride, with release of fatty acids and glycerol. However, within brown adipocytes, most fatty acids are immediately oxidized in mitochondria and, because of the uncoupling protein, a large amount of heat is produced. [Show More]
Last updated: 3 years ago
Preview 1 out of 12 pages
 (1).png)
Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Also available in bundle (1)
Click Below to Access Bundle(s)

BIOL 3362 - Exam 1 -4 Study Guide BUNDLE | Download To Score A.
BIOL 3362 - Exam 1 -4 Study Guide BUNDLE | Download To Score A.
By Prof. Goodluck 4 years ago
$14
5
Reviews( 0 )
$13.50
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Sep 22, 2021
Number of pages
12
Written in
All
Additional information
This document has been written for:
Uploaded
Sep 22, 2021
Downloads
0
Views
119


 And LETRS Unit 8 Final Assessment Test.png)