Chemistry > QUESTIONS & ANSWERS > CHEM 005 Midterm Exam 2 Answers (Penn State University) (All)
CHEM 005 Midterm Exam 2 Answers (Penn State University)
Document Content and Description Below
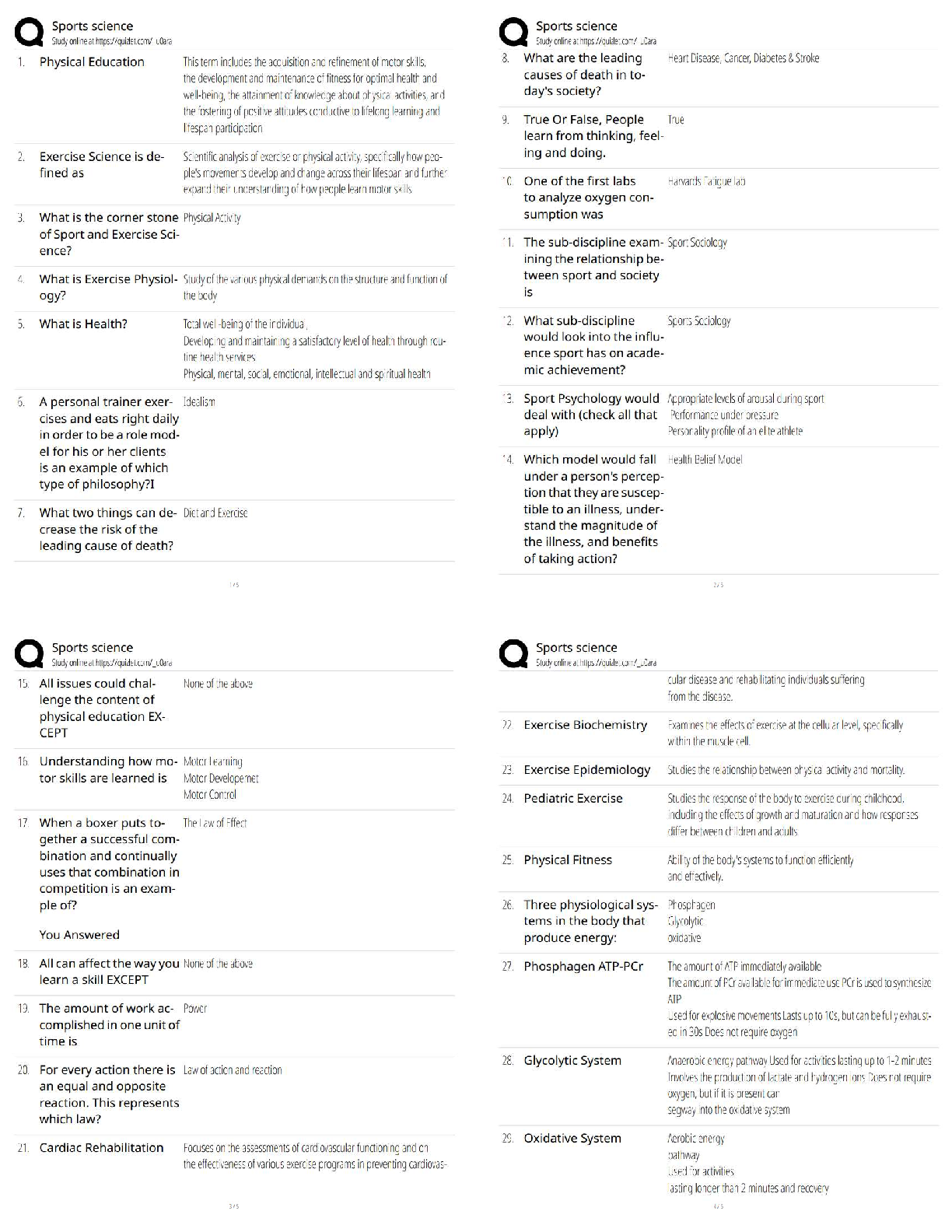
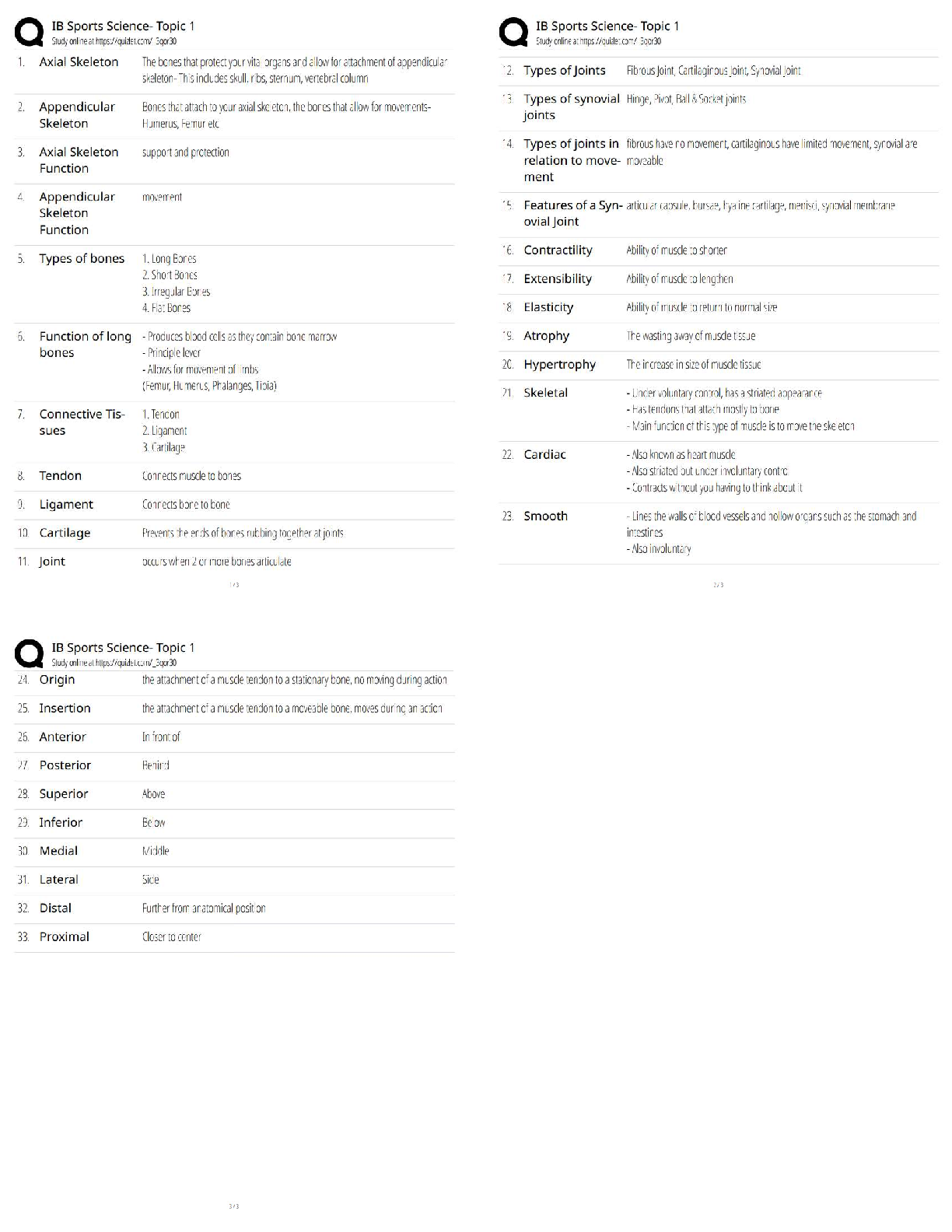
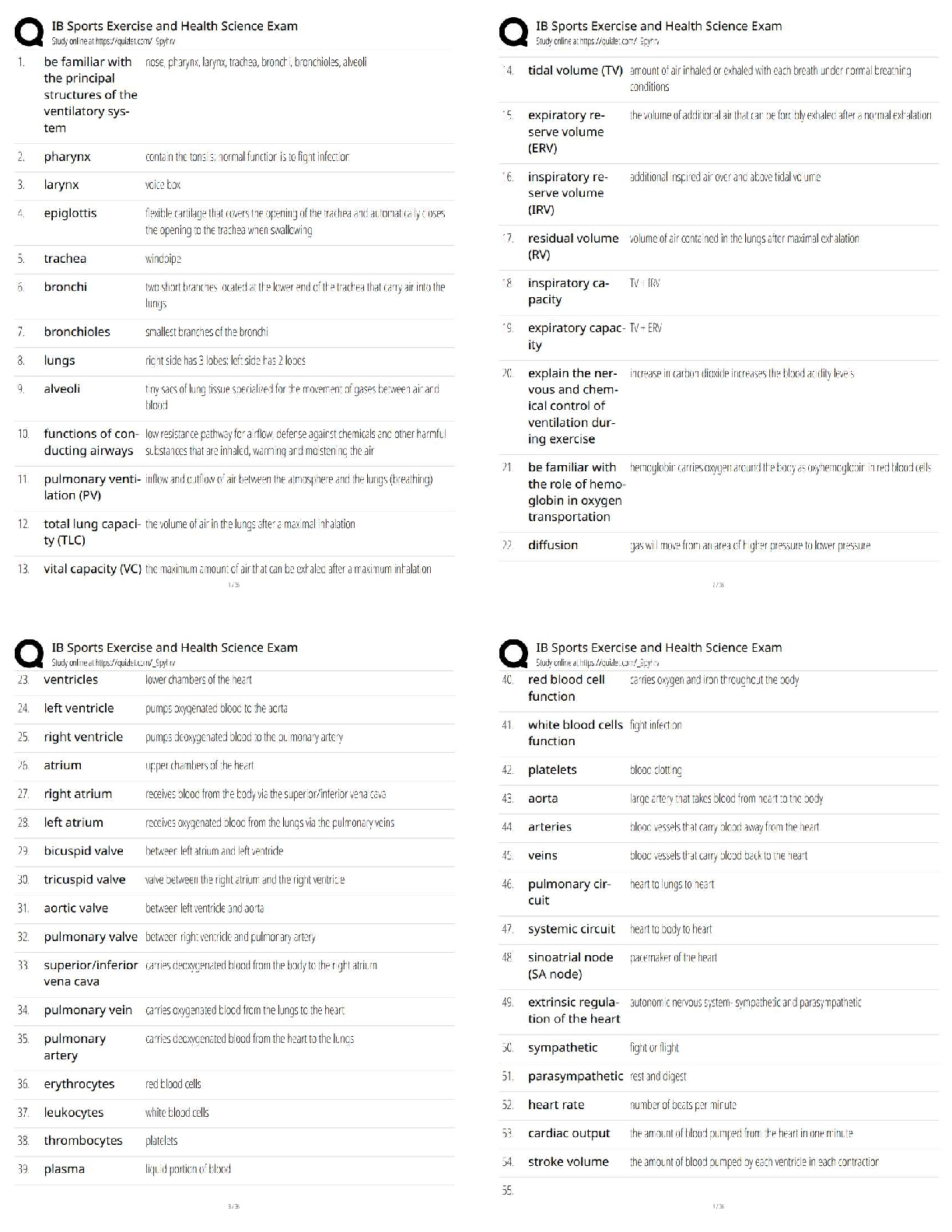
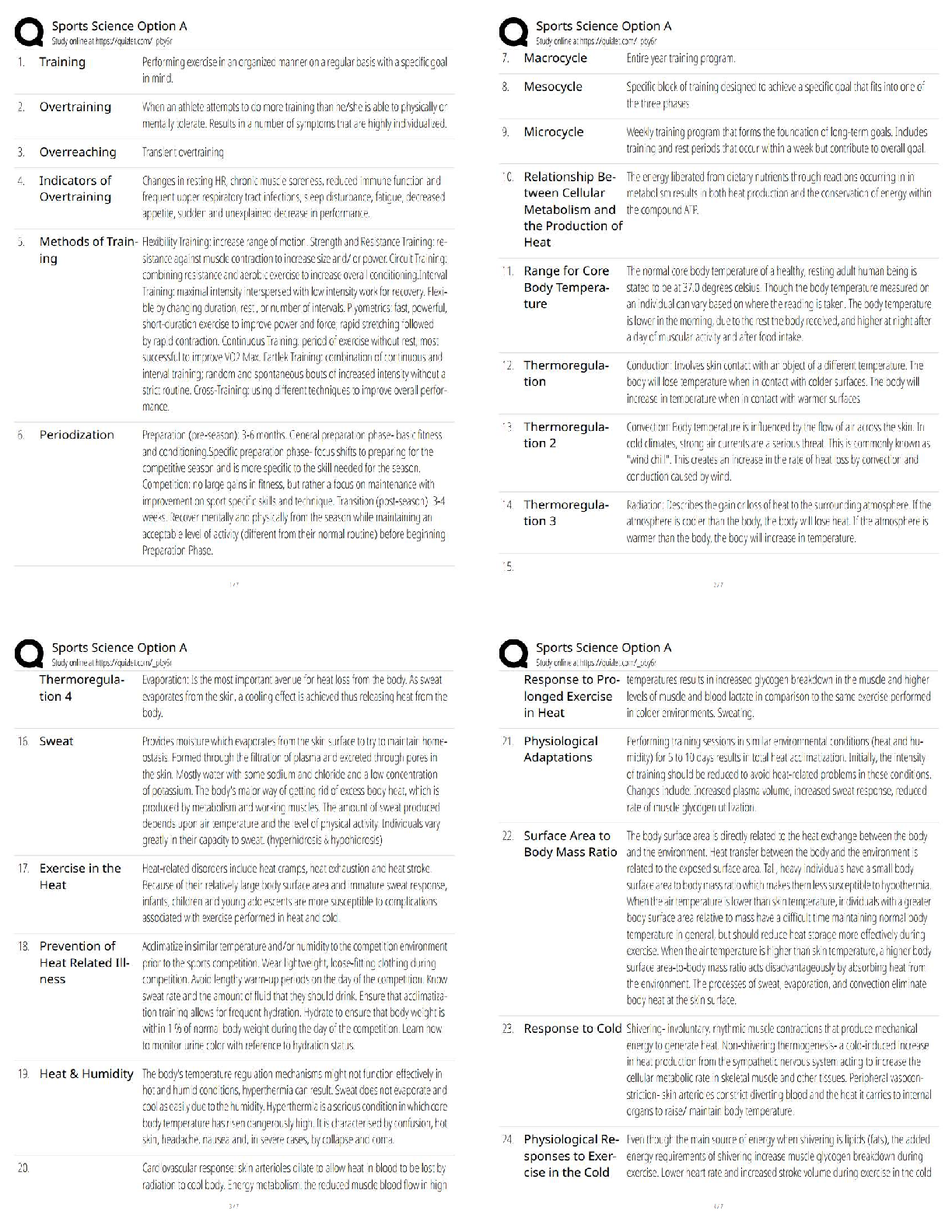
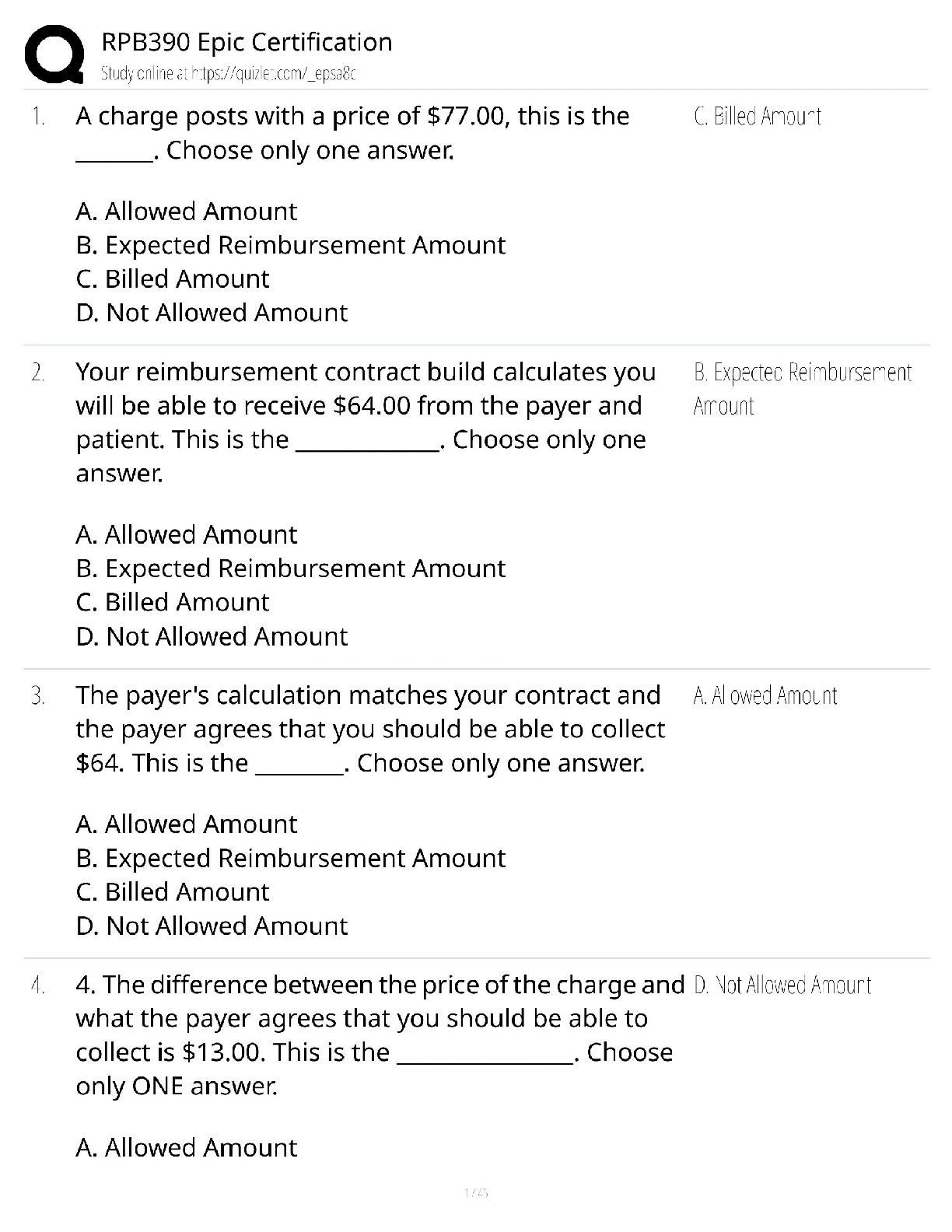
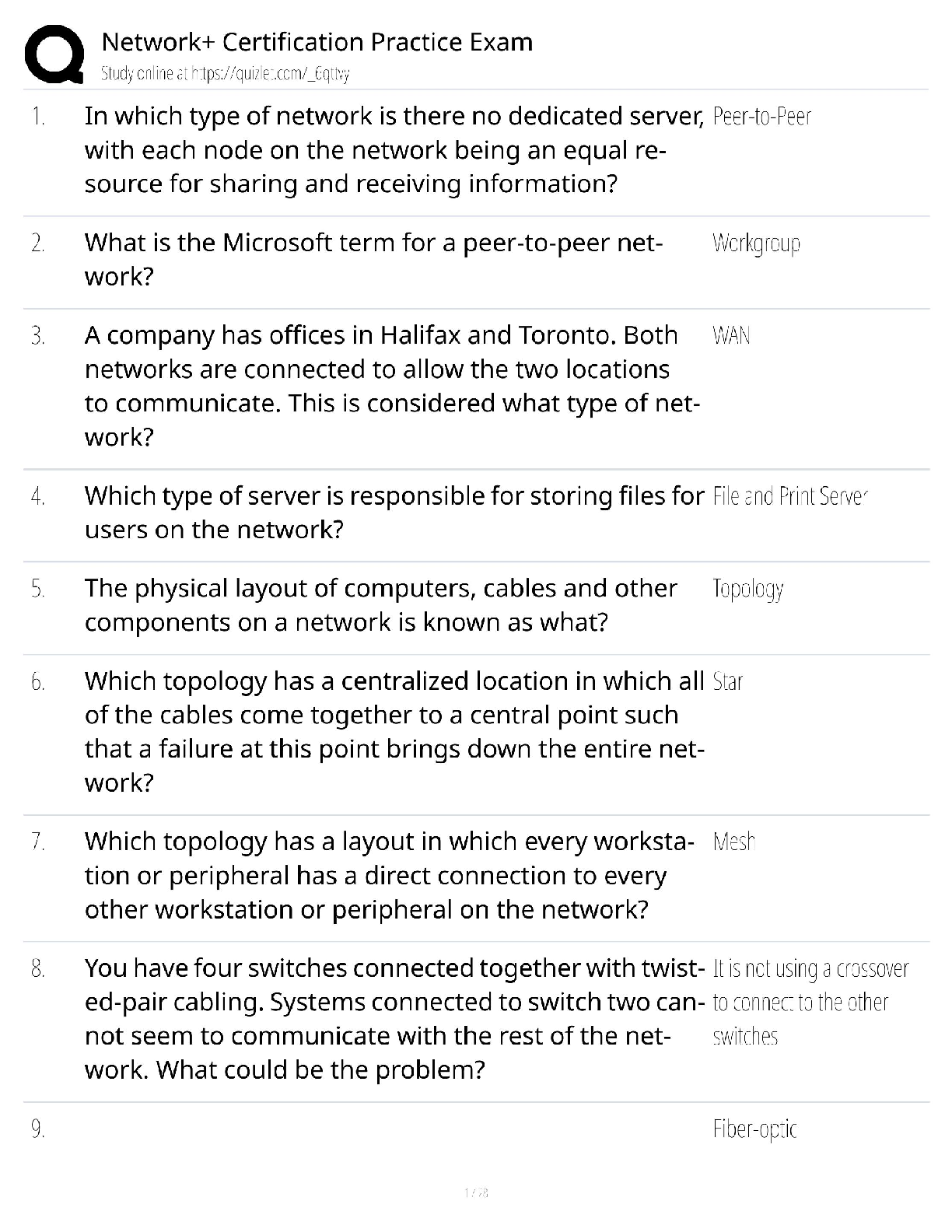
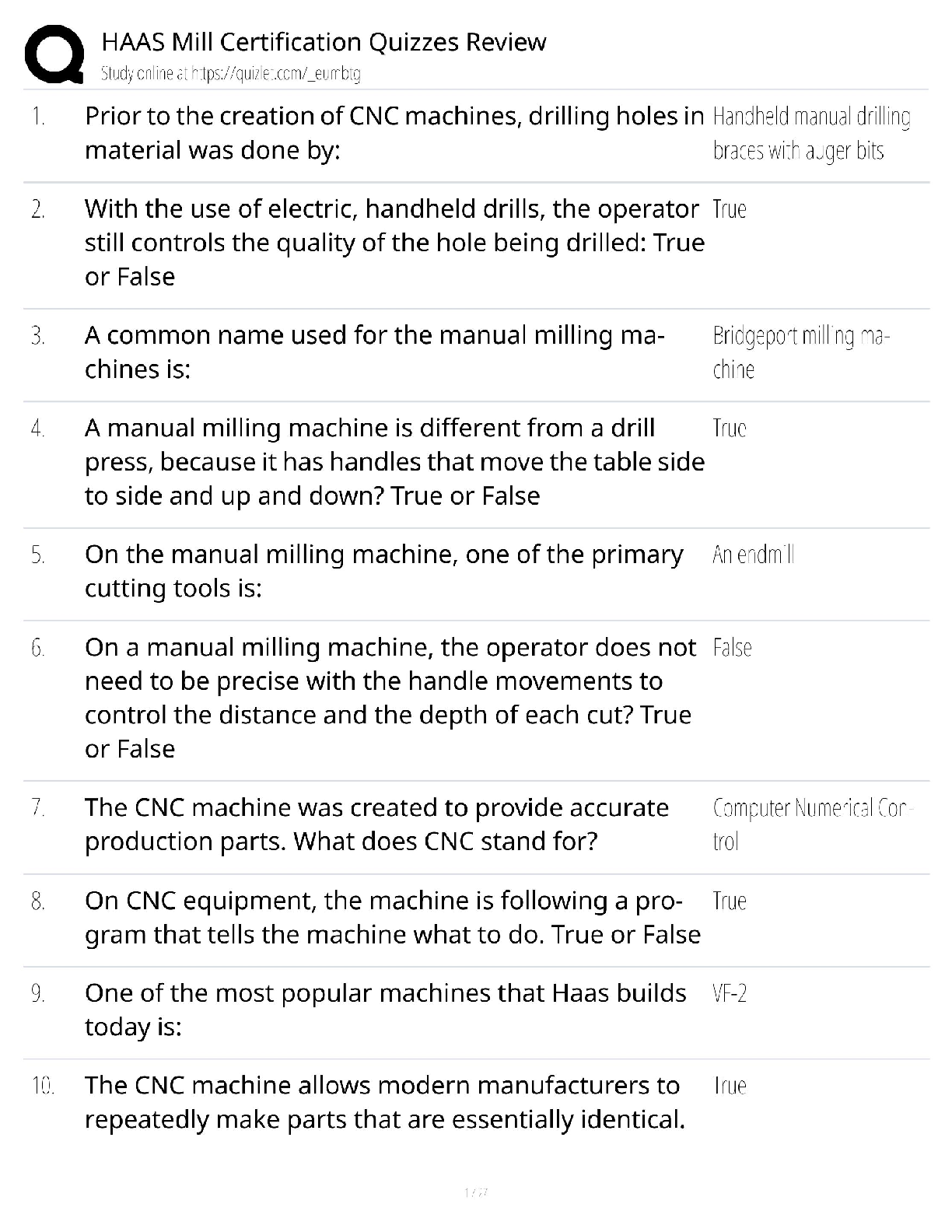
Hard-boiled eggs are made by placing eggs in boiling water until their whites and yolks solidify. Why does hard-boiling an egg take longer at high altitude than at sea level? On a phase diagram, th ... e melting point is the same as __________. Which of the following does not affect gas pressure? The phase diagram of substance X is shown below. When the temperature is raised from 0 °C to 100 °C at 0.25 atm pressure, what phases will be observed during this temperature change? (hint: please note the temperature scale on the diagram is in K). The recommend bike tire pressure for mountain bike tires is about 40 psi. What is this pressure in atmosphere? How does a pressure cooker change the boiling point of water? Recipes often call for the addition to salt to water before you put the pasta in. If a change in boiling point occurs, is it sufficiently large to make a difference when cooking pasta? The boiling point of water in a sugar water solution increases with the concentration of the sugar. Which of the following processes is incorrectly matched with its phase transition? Cooking food with heat is usually a reversible process. A simple pH indicator can be made by soaking red cabbage or red onions in boiling water for a few minutes, then collecting the liquid. This process extracts dye molecules called cyanidins, which change color between blue and red, according to the reaction summarized below: Cyanidin (red) ⇌ Cyanidin (blue) + H+ Which color will the pH indicator turn as a solution of baking soda is added? Sucrose from fruits and vegetables is healthier than refined sucrose. The substance that holds the plant cells together and is largely responsible for fruit and vegetable texture is known as The pigments known as anthocyanins are found in Sulfur compounds are responsible for the flavors and aromas in the onion family of vegetables. With prolonged cooking, the aromas in the onion family become milder and milder. The monomers for lignin are shown above. What functional groups are present in all three monomers? Which statements about viscosity are true? i. Viscosity inceases as temperature decreases. ii. Viscosity increases as intermolecular forces increases. Which of the following is a good way to measure viscosity? Check all that apply. In what ways do the polysaccharides amylose and cellulose differ? Out the of the three liquids listed below, which is the most viscous? which substance do you think would be the more effective thickener? The following factors either decrease the viscosity of a starch paste or delay the gelatinization of a starch paste: Some common thickening agents used in cooking are listed below:Xanthan Gum, Gelatin, Pectin, Alginate, Starch, Flour. What do these molecules have in common? Xanthan gum is a polymer of the form (C35H49O29)n, i.e. there are n repeating units of the basic monomer C35H49O29.What is the molar mass (in g/mol) of the monomer? Which of the following fatty acids molecules make up cocoa butter? i. oleic acidi i. steric acid iii. palmitic acid What is conching? Dutch-processed cocoa powder is usually paired with baking powder What is the ideal polymorph of chocolate? What is the chemical in chocolate that is toxic to dogs and cats? A chemical that makes us feel “blissful” and is produced naturally by the brain, is also found in chocolate. [Show More]
Last updated: 3 years ago
Preview 1 out of 14 pages
.png)
Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$18.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Sep 22, 2020
Number of pages
14
Written in
All
Additional information
This document has been written for:
Uploaded
Sep 22, 2020
Downloads
0
Views
138












 Questions and Answers (latest Update), All Correct, Download to Score A.png)