Health Care > QUESTIONS & ANSWERS > Practice Questions EXAM MATERIAL questions and answer review 2020 docs (All)
Practice Questions EXAM MATERIAL questions and answer review 2020 docs
Document Content and Description Below
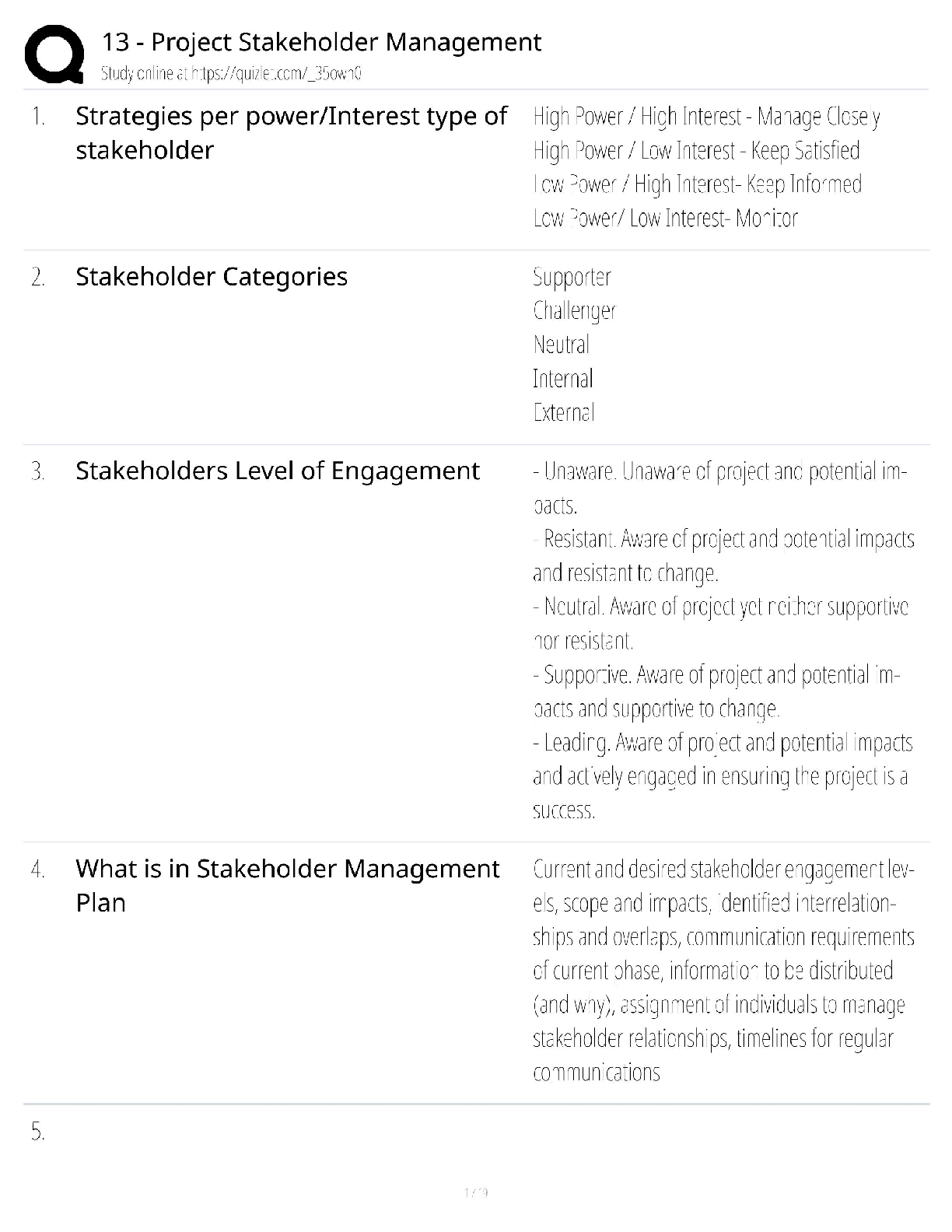
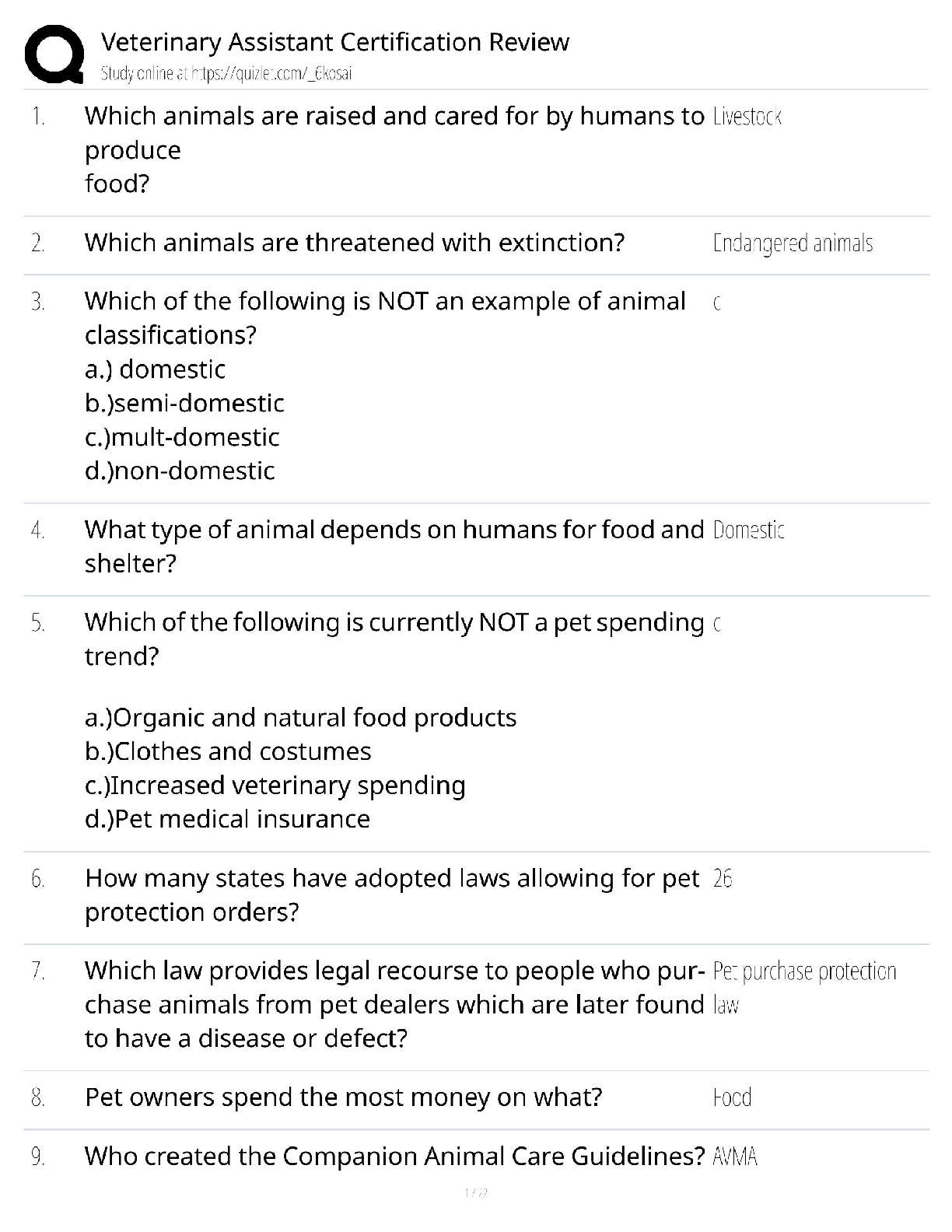
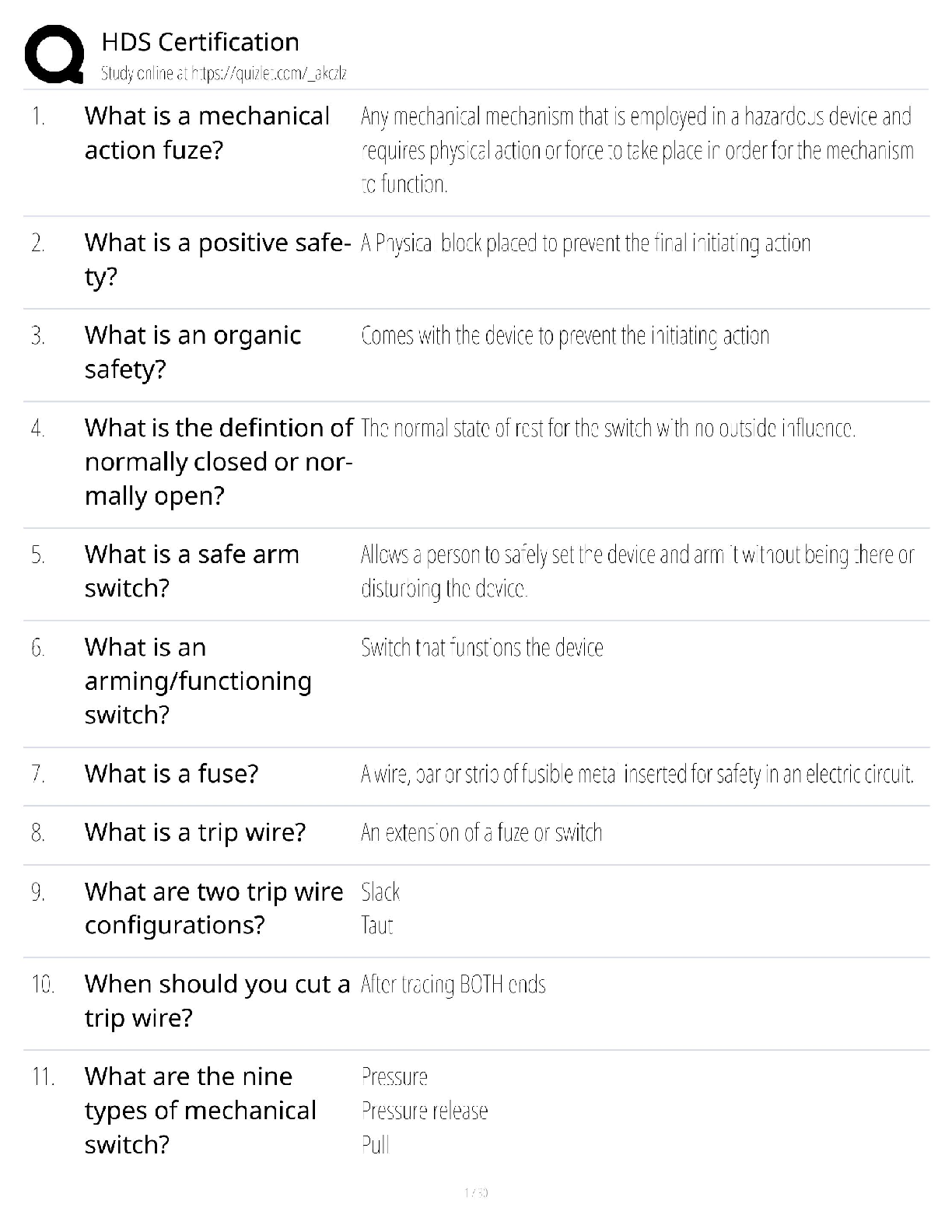
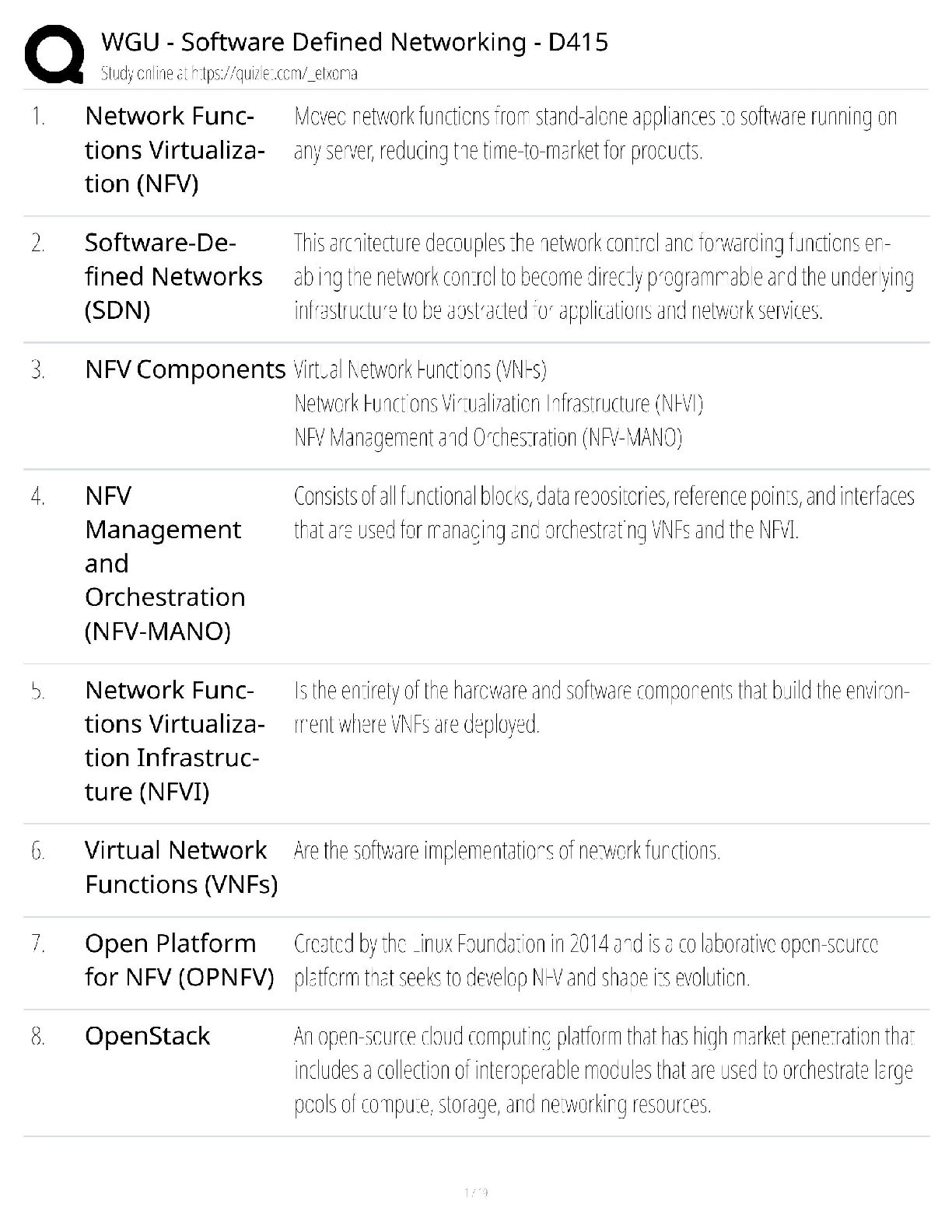
Practice Questions EXAM MATERIAL questions and answer review 2020 docs 1) Which of the following is NOT a property of ALL living systems as defined by the textbook? A) the ability to compe ... nsate for changes in the external environment B) the ability to engage in metabolic activities C) the possession of chemical instructions that govern their structure and function D) the ability to gain energy through consumption of other living things 2) Which of the following details the typical path taken by a protein that is going to be secreted? A) cytoplasm secretory vesicle golgi cell surface B) cytoplasm lysosome secretory vesicle cell surface C) ER secretory vesicle golgi cell surface D) ER golgi secretory vesicle cell surface 3) Which of the following is NOT a characteristic of prokaryotes? A) cell wall B) cell membrane C) endoplasmic reticulum D) DNA 4) What is the difference in the functioning between rough ER and smooth ER? A) Rough ER makes proteins for use inside the cell, while smooth ER makes proteins for use outside. B) Rough ER makes proteins for use outside of the cell, while smooth ER makes lipids and carbohydrates. C) Rough ER detoxifies poisons, while smooth ER creates new cell organelles. D) Rough ER is used by animal cells, while smooth ER is only used by plant cells. 5) Eukaryotic cells contain the following: A) a nucleus that is not surrounded by a membrane B) structures that specialize in energy production C) all of these answers D) circular chromosomal structures within a membrane-bound nucleus 6) Which of the following is found both in eukaryotic and prokaryotic cells? A) ribosomes B) mitochondrion C) vacuoles D) nucleus 7) A key role of peroxisomes includes: A) the ability to produce ATP using oxygen-dependent metabolism. B) the ability to congregate in large amounts within cancer cells to neutralize them. C) the ability to perform lipid metabolism and chemically neutralize free radicals. D) the ability to transportcargo from the ER to the Golgi. 8) Rank the following bonds in order of increasing strength: A) ionic bonds B) covalent bonds C) hydrogen bonds A) C < B< A B) B < A < C C) A < B < C D) B < C < A 9) Which of the following statements correctly describes the relative masses of the three subatomic particles? A) Protons and neutrons weigh the same, electrons weigh much less. B) Neutrons and electrons weigh the same, protons weigh much less. C) Protons and electrons weigh the same, neutrons weigh much less. D) Protons, neutrons and electrons all weight the same. 10) Which of the following statements correctly describes the relative charges of the three subatomic particles? A) Protons and neutrons have a charge of +1, while electrons have a much smaller charge. B) Neutrons are positive, electrons are neutral, protons are negative. C) Electrons are positive, protons are neutral, neutrons are negative. D) Protons are positive, neutrons are neutral, electrons are negative. 11) Which of the following statements accurately describes the locations of the three subatomic particles that make up an atom? A) The protons and electrons are in the nucleus, while the neutrons orbit the nucleus. B) The protons, neutrons and electrons are all in the nucleus. C) The neutrons and electrons are in the nucleus, while the protons orbit the nucleus. D) The protons and neutrons are in the nucleus, while the electrons orbit the nucleus. 12) Which is not a characteristic of hydrogen bonds? A) The hydrogen atom participating in the hydrogen bond must be covalently bounded to a very electronegative atom B) The hydrogen bonding results in higher boiling point, higher melting point, and higher heat of vaporization. C) The hydrogen attaches to the other atom through Van der Waals forces. D) The other atom involved in the hydrogen bond (not the hydrogen) must have at least one lone pair of electrons 13) Dehydration synthesis leads to the formation of which of the following? A) water and monomers B) water and polymers C) water and a hydroxyl group D) water and amino acids 14) During the breakdown of polymers, which of the following reactions takes place? A) covalent bond B) dehydration C) condensation D) hydrolysis 15) Which of the following includes all others? A) Carbohydrate B) Monosaccharide C) Glucose D) Polysaccharide 16) Which of the following describe disaccharides? A) Cellulose is the result of two monosaccharides that have undergone a condensation reaction. B) Glucose, galactose, and fructose are isomers with more than one asymmetric carbon. C) Lactose, maltose, and sucrose are the result of bonded monosaccharides after a dehydration reaction. D) They are a long chain of monosaccharides linked by glycosidic bonds. 17) In terms of their chemical properties, steroids are most similar to: A) Phospholipids B) Polypeptides C) Disaccharides D) Pyrimidines 18) Which of the following is false? A) Starch, glycogen and cellulose are all made of glucose monomers. B) Whether carbon 1 makes an alpha bond or a beta bond depends on the position of the –OH group. C) Chitin and cellulose both have monomers connected by a β-1-to-4 bond D) In starch, Amylose differs from Amylopectin in that Amylose is highly branched and Amylopectin is linear. 19) What is the relationship between a domain and a motif? A) Domain is a structure in a protein with a specific function while a motif is a structural component that makes up the domain B) A motif is the part of the protein with a specific function and a domain is a structural component that makes up the motif C) A motif and a domain both represent structural components of a protein D) A motif and a domain both represent the part of a protein with a specific function 20) Which of the following best describes the phospholipid bilayer? A) The hydrophilic heads are attracted to the intracellular and extracellular fluid of the cell. B) The hydrophobic tails give it a rigid structure to keep it locked in place. C) The hydrophobic tails are positive, and the hydrophilic heads are negative. D) The hydrophilic heads meet in the inner region of the membrane. 21) Which of the following protein structures forms primarily because of hydrogen bonds? A) The tertiary structure B) α-helices and β-pleated sheets C) The primary structure D) The combination of multiple polypeptide subunits 22) When a protein denatures, which of the following protein structures is unaffected? A) Secondary structure B) Tertiary structure C) Primary structure D) Quaternary structure 23) The amino acid Lysine belongs to what group of amino acids? A) nonpolar B) uncharged polar C) negatively charged (acidic) polar D) positively charged (basic) polar 24) Which of the following regarding DNA structure is TRUE? A) A purine must bind with a pyrimidine to ensure a uniform diameter B) DNA does not have a uniform diameter C) A pyrimidine must bind with a pyrimidine to ensure a uniform diameter D) A purine must bind with a purine to ensure a uniform diameter 25) Which method of replication would result in a DNA strand that contained fragments from both the parent strand and newly-synthesized portions? A) Conservative replication B) Semi-conservative replication C) Dispersive replication D) Interspersed replication 26) Which key factors were utilized by Meselson and Stahl to show DNA replication followed a semi-conservative method? A) Nitrogen isotopes B) Radiolabeled deoxyribose C) Phosphorus isotopes D) Radiolabeled phosphate groups 27) Which of the following events is matched correctly to its corresponding stage? A) elongation: replication forks are formed in the DNA helix B) initiation: nucleotides are added to the DNA backbone C) all of these answers D) termination: RNA primer is replaced by DNA nucleotides 28) In the absence of telomerase, which of the following is likely to result? A) Telomeres elongate since DNA replication can finally take place. B) DNA replication and repair continues normally. C) Telomeres continue to shorten since 5’ end DNA cannot be replicated. D) DNA polymerase takes over replicating and repairing the telomeres. 29) The first mechanism of examining DNA for nucleotide errors is: A) nucleotide excision repair B) DNA polymerase proofreading C) UV exposure D) mismatch repair 30) Labeling of viral coats and genetic material in the Hershey-Chase experiment took advantage of the fact that ________ groups are found in nucleic acids, but not in amino acids, and that _______ groups are found in amino acids, but not in nucleic acids. A) Carbonyl; amino B) Phosphate; sulfhydryl C) Carbonyl; sulfhydryl D) Phosphate; amino 31) The Central Dogma of Molecular Biology states that genetic information flows in which of the following sequences? A) DNA to RNA to protein. B) Protein to DNA to RNA. C) Protein to RNA to DNA. D) RNA to DNA to protein. 32) How many nucleotides are in 12 mRNA codons? A) 24 B) 36 C) 48 D) 12 33) Where is a promoter located relative to the initiation site? A) Downstream of the initiation site B) Upwind of the initiation site C) Downwind of the initiation site D) Upstream of the initiation site 34) What are the three major steps of transcription? A) Nucleus, Endoplasmic Reticulum, Ribosome B) Initiation, Elongation, Termination C) RNA Polymerase I, RNA Polymerase II, RNA Polymerase III D) DNA, mRNA, protein 35) Which of the following accurately outlines the order of mRNA processing following transcription? A) The poly(A) tail is added first, followed by adding the 5' cap, and finally the pre-mRNA is spliced. B) The 5' cap is added first, followed by splicing the pre-mRNA, and finally the poly(A) tail is added. C) The 5' cap is added first, followed by the poly(A) tail, and finally the pre-mRNA is spliced. D) The pre-mRNA is spliced first, followed by adding the 5' cap, and finally the poly(A) tail is added. 36) During translation, the completed peptide chain exits the ribosome from the A) E site. B) P site. C) small subunit. D)A site. 37) Which of the following statements accurately describes the role ribosomes play in protein synthesis? A) Ribosomes read mRNA and use tRNAs to produce peptides and proteins. B) Ribosomes carry individual amino acids and load them onto growing peptide chains. C) Ribosomes transport mRNA chains from the nucleus to the cytoplasm. D) Ribosomes load amino acids onto tRNAs. 38) Which of the following about tRNAs is true? A) Each organism only expresses one tRNA molecule for translation. B) Elongation factors charge tRNAs with amino acids at the 3' end. C) tRNA molecules can be charged with any amino acid. D) Charged tRNAs bind to mRNA through three nucleotide base pairings. 39) Many antibiotics inhibit bacterial protein synthesis. For example, chloramphenicol blocks peptidyl transfer. What specific effect would you expect this antibiotic to have on protein synthesis? A) Chloramphenicol would directly affect tRNA binding to the ribosome. B) Chloramphenicol would directly affect growth of the protein chain. C) All of these answers. D) Chloramphenicol would directly affect ribosome assembly. 40) All of the following post-translational events are critical for proper protein function EXCEPT: A) chemical modification of amino acid residues B) targeting of the protein to a subcellular location C) denaturation of the protein to disconnect it from the ribosome D) folding of the protein into the proper three-dimensional structure 41) During which process is a new copy of DNA is synthesized? A) Translation B) Transcription C) Translocation D) Replication 42) Proofreading activity in DNA synthesis: A) occurs after the synthesis is complete B) is a function of the 3'-5' exonuclease activity of DNA Polymerase C) requires a separate enzyme from DNA polymerase D) occurs only in the DNA synthesis from the lagging strand 43) Which enzyme unwinds short stretches of DNA helix ahead of the replication fork? A) DNA Polymerase B) Helicase C) Single-stranded binding proteins D) Topoisomerase 44) Which of the following is NOT a factor in the formation of an initiation complex for eukaryotic translation? A)ATP B) initiator tRNA C) GTP D) IF 45) Based on what you know about the pH scale, a weakly acidic apple would likely have a value of: A) 0 B) 2 C) 4 D) 9 46) Which of the following structures is formed from the interactions of the amino acid R groups? A) Secondary structure B) Tertiary structure C) Quaternary structure D) Primary structure 47) Which of the following best describes why humans have a similar gene number as do nematodes despite being a more complex organism? A) Splicing removes introns from the human genes to efficiently produce proteins. B) Nematodes have an excess of exons that are not used to produce proteins. C) Splicing removes introns from the nematode genes to efficiently produce proteins. D) Alternative splicing occurs in almost all human genes to produce multiple proteins. 48) Which chemical interactions are most responsible for the secondary structure of proteins? A) Covalent bonds B) Hydrogen bonds C) Van der Waals forces D) All of the above 49) What was the main conclusion of the Hershey and Chase experiment using phage T2? A) Proteins and DNA can be distinguished because proteins have sulfur and DNA has phosphorus B) Cells can uptake a transforming principle released by the breakdown of other cells, known as transformation. C) DNA is the hereditary molecule D) Protein is the hereditary molecule 50. Which of the following is FALSE about the genetic code? A) The genetic code is degenerate but not ambiguous B) The start codon always codes for methionine C) 1 amino acid can be coded by more than 1 codon D) 1 codon can code more than 1 amino acid 51) One of the many functions of proteins is to regulate gene expression by binding to specific regions on a DNA molecule. DNA has a negative charge, due to the phosphate groups that form the backbone of nucleic acids. In a protein that binds to DNA, which aminoacid below would be most likely to mediate the interaction with DNA? A) Glutamic acid B) Proline C) Lysine D) Valine 52) Why is it critical that an aminoacyl tRNA synthetase recognizes a specific tRNA molecule? A) Each tRNA with the proper anti-codon sequence must be charged with the correct amino acid B) Each tRNA must be properly folded by a tRNA synthetase enzyme C) Each tRNA needs to be labeled at the 5' terminus with the correct anti-codon D) A tRNA will not bind to the ribosome without its corresponding tRNA synthetase 53) Cyclohexane (C6H12 in a ring) and benzene (C6H6 in a ring) are considered _______ of each other. A) Polymers B) Isomers C) Isotopes D) None of the above 54) Vegetable oils are typically liquid at room temperature, but can be made to become solid (as in the production of margarine) by: A) Converting them into phospholipids B) Forming carbon-carbon double bonds C) Converting them into neutral lipids D) Adding hydrogens to make them saturated 55) If an atom of oxygen has 8 protons, then molecular oxygen has an atomic mass of: A) 8 B) 12 C) 32 D) 16 56) The amino acid tyrosine belongs to what group of amino acids? A) nonpolar B) polar uncharged C) polar negatively charged D) polar positively charged 57) Which of the following does the enzyme primase synthesize? A) phosphodiester linkage B) DNA primer C) RNA primer D) Okazaki fragments 58) The two theories concerning eukaryotic evolution largely differ in their explanation of: A) the resolution of the stalemate between the archaean and bacterial cells involved B) the nature of the ultimate relationship between the mitochondria and the host cell in the resulting eukaryotic cell C) the mechanism through which the endomembrane system developed D) the precursor of the cell giving rise to the nucleus 59) The DNA polymerase, RNA polymerase, and Ribosome READ their respective templates in the: A) 3’ to 5’, 3’ to 5’, 5’ to 3’ directions respectively. B) 3’ to 5’, 5’ to 3’, 5’ to 3’ directions respectively. C) in the 3’ to 5’ direction. D) 5’ to 5’, 3’ to 5’, 5’ to 3’ directions respectively. 60) What would likely happen if an aminoacyl synthetase gene was mutated and nonfunctional? A) proteins would be made with incorrect amino acids incorporated B) only truncated proteins would be made C) some truncated proteins would be made when a specific amino acid was required D) protein synthesis would not occur 61) Which of the following is NOT a role of the ribosome during translation? A) catalyze reaction with peptidyl transferase B) read amino acids C) bind mRNA D) bind tRNA 62) In order to perform their jobs, RNA Polymerase II and DNA Polymerase III both need: A) a helicase B) primase C) a DNA polymer D) an RNA polymer 63) The following amino acids are most likely found on the surface of a folded globular protein EXCEPT for ______. A) Serine B) Proline C) Arginine D) Tyrosine 64) Which of the following is NOT a factor in the formation of an initiation complex for eukaryotic translation? A) ATP B) initiator tRNA C) GTP D) IF 65) Nonpolar organic molecules are good examples of ______. A) electrolytes B) dipole molecules C) hydrophobic compounds D) hydrophilic compounds 66) Which of the following are needed to form a triglyceride molecule? A) 3 glycerol molecules B) 3 fatty acid molecules C) 3 glycerol molecules and 3 fatty acid molecules D) 3 fatty acid molecules and 1 glycerol molecule 67) Which of the following is NOT associated with the endomembrane system? A) Lysosome B) Exocytosis C) Protein synthesis by free ribosomes D) Plasma membrane renewal 68) Which bonds maintain the primary structure of a protein? A) disulfide bonds B) phosphodiester bonds C) hydrogen bonds D) peptide bonds 69) Which of the following functional group(s) may be used to form polymers via dehydration reactions? A) only hydroxyl groups B) only carbonyl groups C) either carboxyl or carbonyl groups D) either hydroxyl or carboxyl groups 70) If the antisense strand of a DNA molecule has the sequence of bases 5'-ATTGCA-3', the transcript would have the sequence ______. A) 5-'TAACGT-3' B) 5'-TGCAAT-3' C) 5'-UAACGU-3' D) 5'-UGCAAU-3' 71) Which is NOT different between prokaryotic and eukaryotic transcription? A) Termination B) Requirement for transcription factors to start C) Protein responsible for unwinding DNA D) RNA processing 72) Polypeptides newly synthesized by ribosomes could be found in the following subcellular space EXCEPT for ______. A) cytoplasm B) lumen of rough ER C) matrix of mitochondria D) nucleus 73) Cytosine makes up 42% of the nucleotides in a sample of DNA from an organism. Approximately what percentage of the nucleotides in this sample will be thymine? A) 8% B) 16% C) 31% D) 42% 74) During splicing, which molecular component of the spliceosome catalyzes the excision reaction? A) protein B) DNA C) RNA D) lipid 75) Which of the following is a true statement? A) a motif is the same as a domain B) a domain can contain a motif C) a motif can contain a domain D) both B and C are true 76) Which of the following is NOT a property of living systems: A) Living Systems contain Chemical Instructions for Structure and Function B) Living Systems have energy and matter flow through them C) Living systems are all multi-cellular D) Living systems reproduce 77) Which of the following about Prokaryotic cell structures is FALSE A) They have no internal membrane bound organelles B) They have a low Surface Area to Volume Ratio C) They are not complex D) They have one single chamber 78) The ___ is a membrane bound organelle that is involved in the metabolism of lipids A) mitochondria B) nucleus C) endoplasmic reticulum D) peroxisome 79) Which subatomic particle/s account for the atomic mass A) Electron B) Proton + electron C) Electron+ neutron D) Proton+neutron 80) Which of the following Atoms has the highest electronegativity? A) Cl B) Na C) Si D) S 81) How are the two compounds below related? A) Enantiomers B) Isomers C) Aldehydes D) Monosaccharides 82) The formation of a triglyceride involves the process of: A) Hydrolysis B) Condensation C) Glyceration D) Fatty acid cosynthesis 83) Which of the following Amino Acids is NOT a Nonpolar Amino Acid A) Methionine B) Tryptophan C) Lysine D) Leucine 84) What special property can the amino acid Cysteine confer on a protein’s structure? A) The cysteine makes it work twice as fast as other enzymes B) The cysteine gives it beta sheets that cause the molecule to glow under the microscope C) The cysteine molecule allows the protein to keep changing forms by being very flexible and unstable. D) The cysteine helps forms di-sulfide bonds with other cysteine molecules in a protein 85) Which functional domain is responsible for proof reading the replicated strand of DNA? A) 3’-5’ exonuclease B) DNA Helicase C) 5’-3’ exonuclease D) Polymerase 86) What did Hershey and Chase conclude from their experiments? A) They concluded that dead, non-infective R cells can change living R cells and cause them to become virulent B) They concluded that DNA is the substance that causes bacterial transformation C) They concluded that protein, and not DNA, was the genetic material D) They concluded that DNA, and not protein, was the genetic material. 87) Which of the following is INCORRECT: A) Guanine is a Purine B) Cytosine is a Pyrimidine C) Adenine is a Pyrimidine D) Uracil is a Pyrimidine 88) Which of the following enzymes is responsible for filling the gaps in the backbone of the DNA strands in Okazaki fragments: A) DNA Polymerase II B) DNA Polymerase III C) DNA Polymerase I D) Topoisomerase 89) The sequence to which RNA Polymerase binds at the start of transcription is found___ A) Downstream of the promoter sequence B) Upstream of the gene to be transcribed C) Within the first intron D) Downstream of the transcription bubble 90) What is the function of snRNPs: A) To add the 5’7-G methyl cap to the mature mRNA B) To recruit RNA Polymerase to the Promotor sequence C) To aid in the polyadenylation of the 3’ end D) To aid in the splicing of introns 91) What two factors aid in the initiation of Translation? A) GTP Hydrolysis and Initiation Factors B) ATP Hydrolysis and Initiation Factors C) GTP Hydrolysis and Codon Recognition Factors D) ATP Hydrolysis and Codon Recognition Factors 92) Which of the below molecules would you NOT expect to find on a phospholipid head group? A) Choline B) Ethanolamine C) Cholesterol D) Inositol 93) Which is true about the two molecules to the right? A) they are stereoisomers B) they are structural isomers C) they are enantiomers D) they are monosaccharides [Show More]
Last updated: 3 years ago
Preview 1 out of 17 pages
.png)
Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$15.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Oct 24, 2020
Number of pages
17
Written in
All
Additional information
This document has been written for:
Uploaded
Oct 24, 2020
Downloads
0
Views
116

 answers.png)