Chemistry > QUESTIONS & ANSWERS > SAM > AS Level Chemistry A_ Breadth in chemistry - Component H032_01. (All)
SAM > AS Level Chemistry A_ Breadth in chemistry - Component H032_01.
Document Content and Description Below
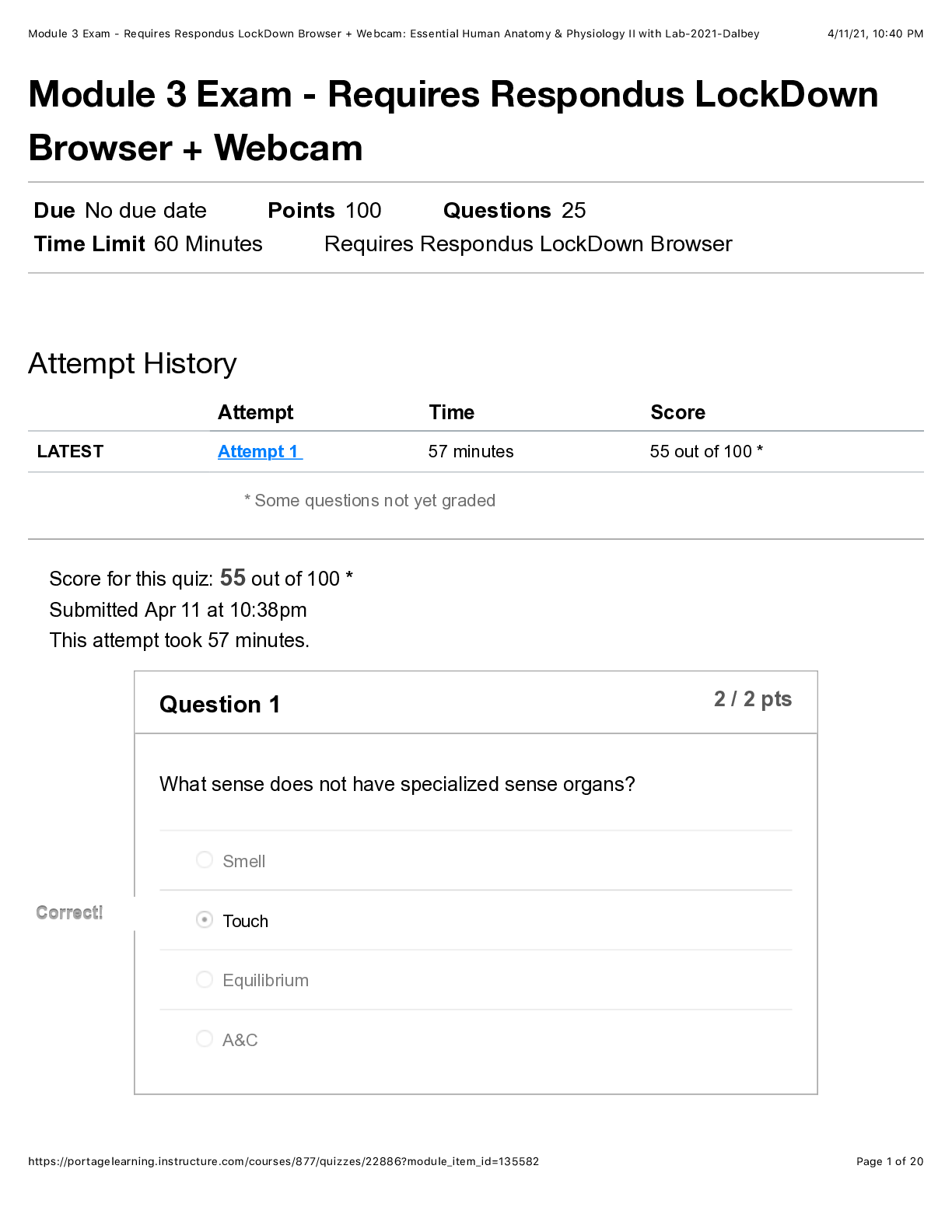
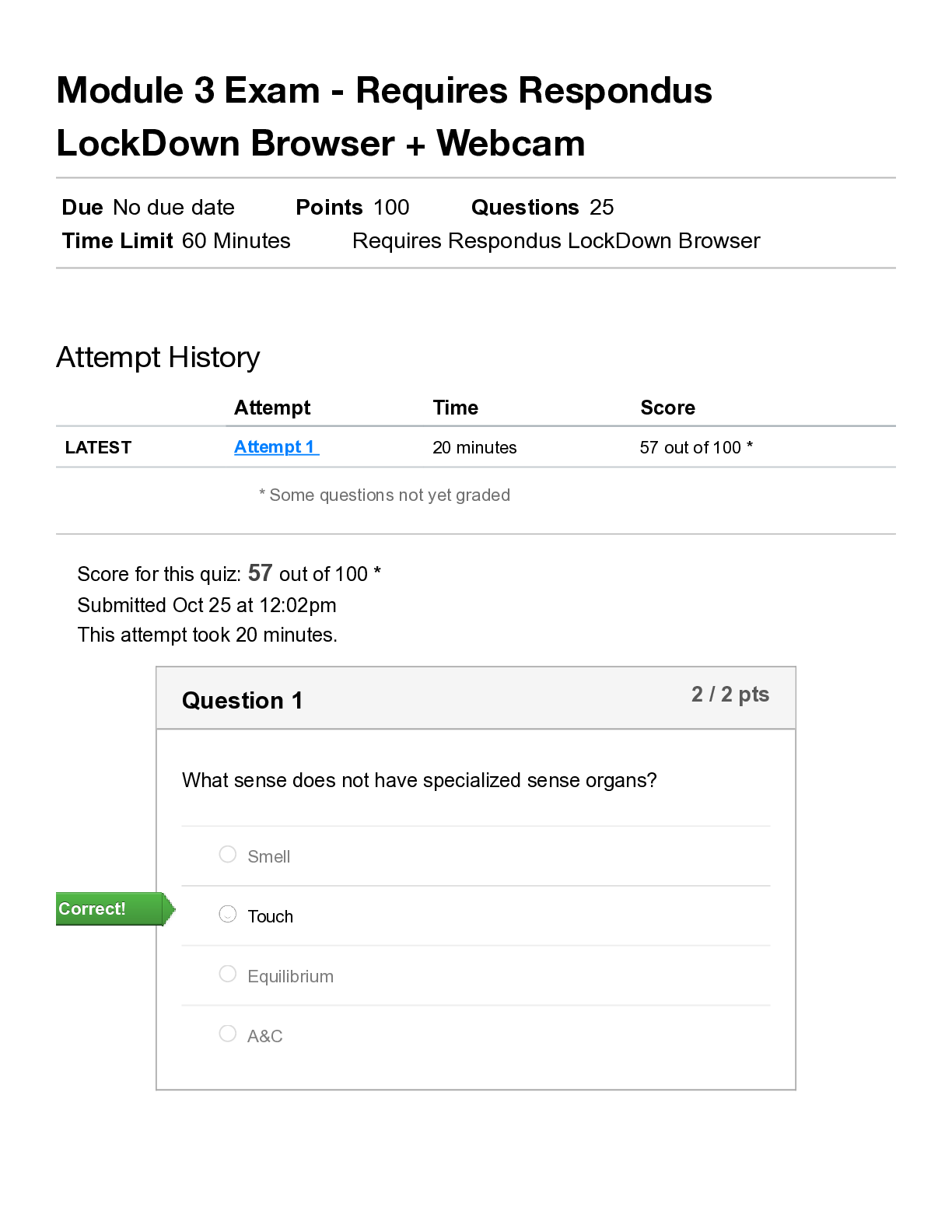
1 How many electrons are in a 12Mg 24 2+ ion? A 10 B 12 C 14 D 22 Your answer [1] 2 What is the formula of chromium(III) sulfate? A Cr3SO4 B Cr(SO4)3 C Cr2(SO4)3 D Cr3SO3 Your answer [1] ... 3 Which molecule is non-polar? A SF6 B H2S C PF3 D NH3 Your answer [1] 3 © OCR 2021 H032/01 Turn over 4 Which row is correct? Highest pH when added to water Most reactive halogen A MgO F2 B MgO I2 C BaO F2 D BaO I2 Your answer [1] 5 Which equation represents a redox reaction? A Mg + 2HCl → MgCl2 + H2 B MgO + 2HCl → H2O + MgCl2 C MgCO3+ 2HCl → CO2 + H2O + MgCl2 D Mg(OH)2 + 2HCl → MgCl2 + 2H2O Your answer [1] 6 This question is about trends in the periodic table. Which trend is correct? A melting point decreases from lithium to carbon B boiling point decreases from fluorine to iodine C first ionisation energy decreases from lithium to caesium D first ionisation energy increases from nitrogen to oxygen Your answer [1] 4 © OCR 2021 H032/01 7 A sample of a compound M contains 1.46 g of carbon, 0.482 g of hydrogen and 1.69 g of nitrogen. What is the empirical formula of M? A CH2N B C4HN4 C CH4N D C2H4N Your answer [1] 8 A student mixes 100 cm3 of 0.200 mol dm–3 NaCl(aq) with 100 cm3 of 0.200 mol dm–3 Na2CO3(aq). What is the total concentration of Na+ ions in the mixture formed? A 0.100 mol dm–3 B 0.200 mol dm–3 C 0.300 mol dm–3 D 0.400 mol dm–3 Your answer [1] 9 Which mass of substance contains the greatest number of atoms? A 3.00 g of ammonia, NH3 B 3.00 g of chloromethane, CHCl3 C 4.00 g of hydrogen sulfide, H2S D 4.00 g of hydrogen chloride, HCl Your answer [1] 5 © OCR 2021 H032/01 Turn over 10 Which reagent would exactly neutralise 100 cm3 of 1.00 mol dm–3 H2SO4(aq)? A 0.100 mol Al(OH)3 B 0.100 mol NH3 C 0.100 mol Ba(OH)2 D 0.100 mol NaOH Your answer [1] 11 The table below shows standard enthalpy changes of formation, ∆fH. Compound NH4NO3(s) H2O(g) CO2(g) ∆fH / kJ mol–1 –366 –242 –394 What is the enthalpy change for the following reaction? 2NH4NO3(s) + C(s) → 2N2 (g) + 4H2O(g) + CO2(g) A –630 kJ mol–1 B –540 kJ mol–1 C +540 kJ mol–1 D +630 kJ mol–1 Your answer [1] 6 © OCR 2021 H032/01 12 Carbon monoxide reacts with steam in the following reaction equation: CO(g) + H2O (g) CO2(g) + H2(g) ∆H = –40 kJ mol–1 Which change will shift the position of equilibrium to the right hand side of the equation? A decrease in pressure B increase in pressure C decrease in temperature D increase in temperature Your answer [1] 13 Which substance contains hydrogen bonding in the liquid state? A CH3(CH2)4CH3 B CH3(CH2)3CHFCH3 C CH3(CH2)3COCH3 D CH3(CH2)3CH(OH)CH3 Your answer [1] 14 Which volume of oxygen gas, at room temperature and pressure, is required for complete combustion of 1.25 × 10−3 mol of propan-1-ol? A 105 cm3 B 120 cm3 C 125 cm3 D 135 cm3 Your answer [1] 7 © OCR 2021 H032/01 Turn over 15 Three of the following displayed formulae represent the same isomer of C3H4Cl2 but one structure represents a different isomer, X. Which displayed formula represents X? A B C D Your answer [1] 16 Which alcohol will not react with potassium dichromate(VI) in sulfuric acid? A CH3CH2CH(OH)CH2CH3 B CH3CH2CH(CH3)CH2OH C (CH3)2CHCH(CH3)OH D (CH3CH2)2C(CH3)OH Your answer [Show More]
Last updated: 3 years ago
Preview 1 out of 44 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$3.50
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
May 07, 2022
Number of pages
44
Written in
All
Additional information
This document has been written for:
Uploaded
May 07, 2022
Downloads
0
Views
121







.png)