BCH 2022 Practice Exam Questions
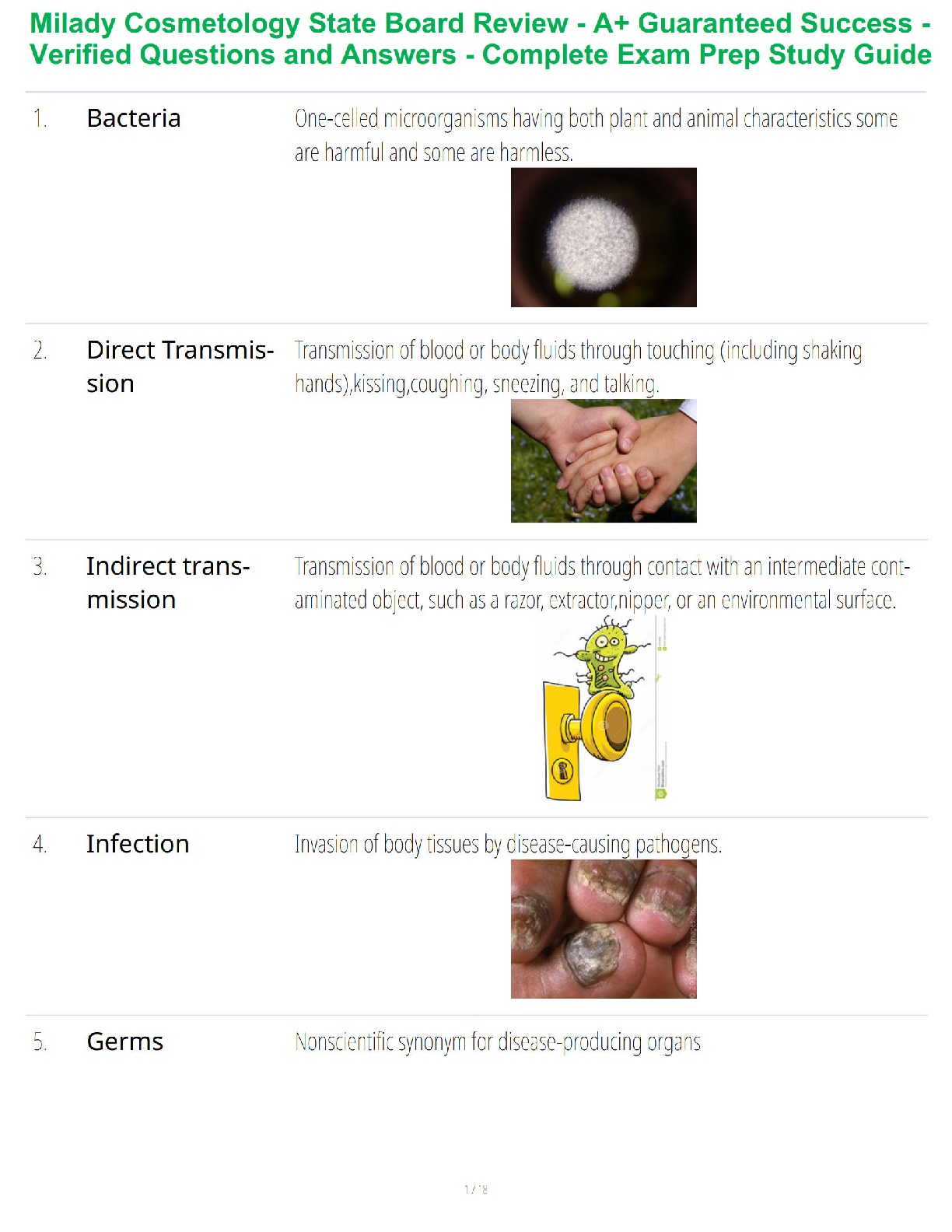
COMPLETE SOLUTIONS
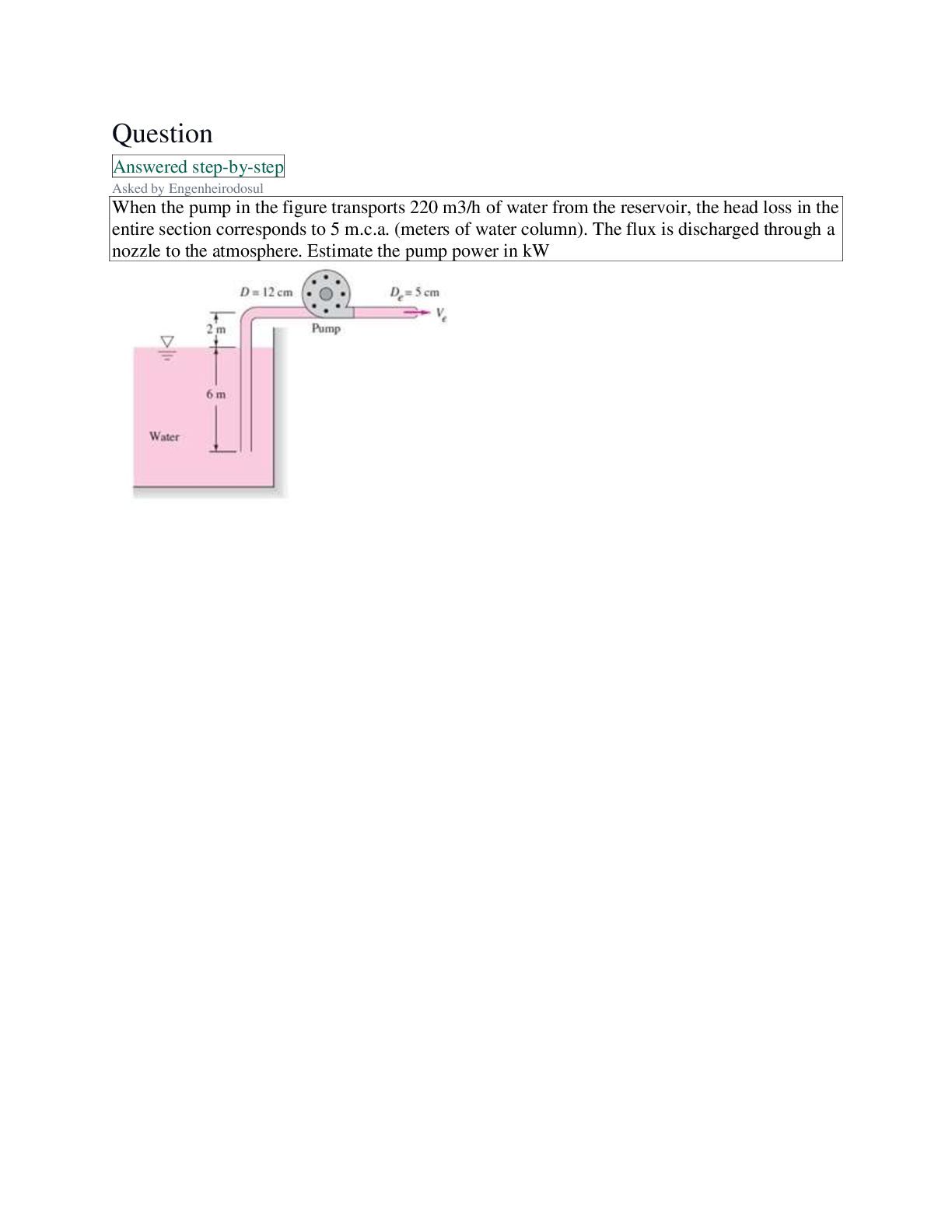
The standard free energy change for the hydrolysis of GTP (guanosine triphosphate) to GDP + Pi is - 7.3
kcal/mol, similar to that for ATP. From this information, onc
...
BCH 2022 Practice Exam Questions
COMPLETE SOLUTIONS
The standard free energy change for the hydrolysis of GTP (guanosine triphosphate) to GDP + Pi is - 7.3
kcal/mol, similar to that for ATP. From this information, once can conclude the
Select one:
a. free energy of GDP and phosphate is higher than the free energy of GTP.
b. reaction is exergonic
c. reaction is endergonic
d. reaction requires energy - ✔✔b. reaction is exergonic
Refer to the figure below.
Which word best fits into this figure where the question mark is located?
Select one:
a. Metabolism
b. Catalysis
c. Energy
d. Equilibrium - ✔✔c. Energy
The high energy phosphate bond in ATP is _____ and ____ energy to break the bond.
Select one:
a. Hard to break, releases
b. Hard to break, requires
c. Easy to break, requires
d. Easy to break, releases - ✔✔c. Easy to break, requires
Match the complexes from the electron transport chain with their direct source of electrons.
Complex III
Complex I
Complex IV
Complex II - ✔✔Complex III -> Ubiquinone/Coenzyme Q
Complex I -> NADH
Complex IV -> Cytochrome C
Complex II -> Succinate/FADH2
Which of the following redox couples has the strongest affinity for electrons?
Select one:
Cytochrome c Fe3+ / cytochrome c Fe2+
O2 / H2O
FAD / FADH2
NAD+ / NADH - ✔✔O2 / H2O
In a theoretical cell type in which has the electron carriers listed below. Based on their standard
reduction potentials (E'°) in the table below. In which order are the electron carriers most likely to act in
carrying electrons?
Oxidant Reductant E`(V)
A+ AH -0.32
B1(oxidised) B2(reduced) -0.62
C3+ C2+ +0.22
D1 D2(oxidised) +0.89
E1(oxidised) E2(FPa)(reduced) +0.77
Select one:
a. D1→ B1 → A+→ C3+ → E1
b. E1 → C3+ → A+ → B1 → D1
c. B1 → A+ → C3+→ E1 → D1
d. B1 → D1→ A+ → C3+ → E1 - ✔✔c. B1 → A+ → C3+→ E1 → D1
Although molecular oxygen does not participate directly in any of the reactions in the citric acid cycle,
the cycle operates only when oxygen is present. This is because oxygen:
Select one:
A. Accepts electrons from the electron transport chain, allowing reoxidation of NADH to NAD+
B. Is necessary for producing water, which is crucial for all cellular processes
C. Removes toxic by-products of the citric acid cycle
D. Is necessary as an activator of enzymes of the cycle - ✔✔A. Accepts electrons from the electron
transport chain, allowing reoxidation of NADH to NAD+
Acetyl CoA
Select one:
A. Undergoes carboxylation to form malate
B. Levels regulate the rate of the citric acid cycle
C. Contains a high energy thioester linkage
D. Activates the enzyme pyruvate dehydrogenase - ✔✔C. Contains a high energy thioester linkage
The Citric Acid Cycle
Select one:
A. Occurs in all cells
B. Is an amphibolic pathway
C. Operates under aerobic and anaerobic conditions
D. Produces 3 FADH molecules, 1 NADH molecule and 1 GTP molecule per every cycle - ✔✔B. Is an
amphibolic pathway
What is the main energy source for the brain?
[Show More]




.png)