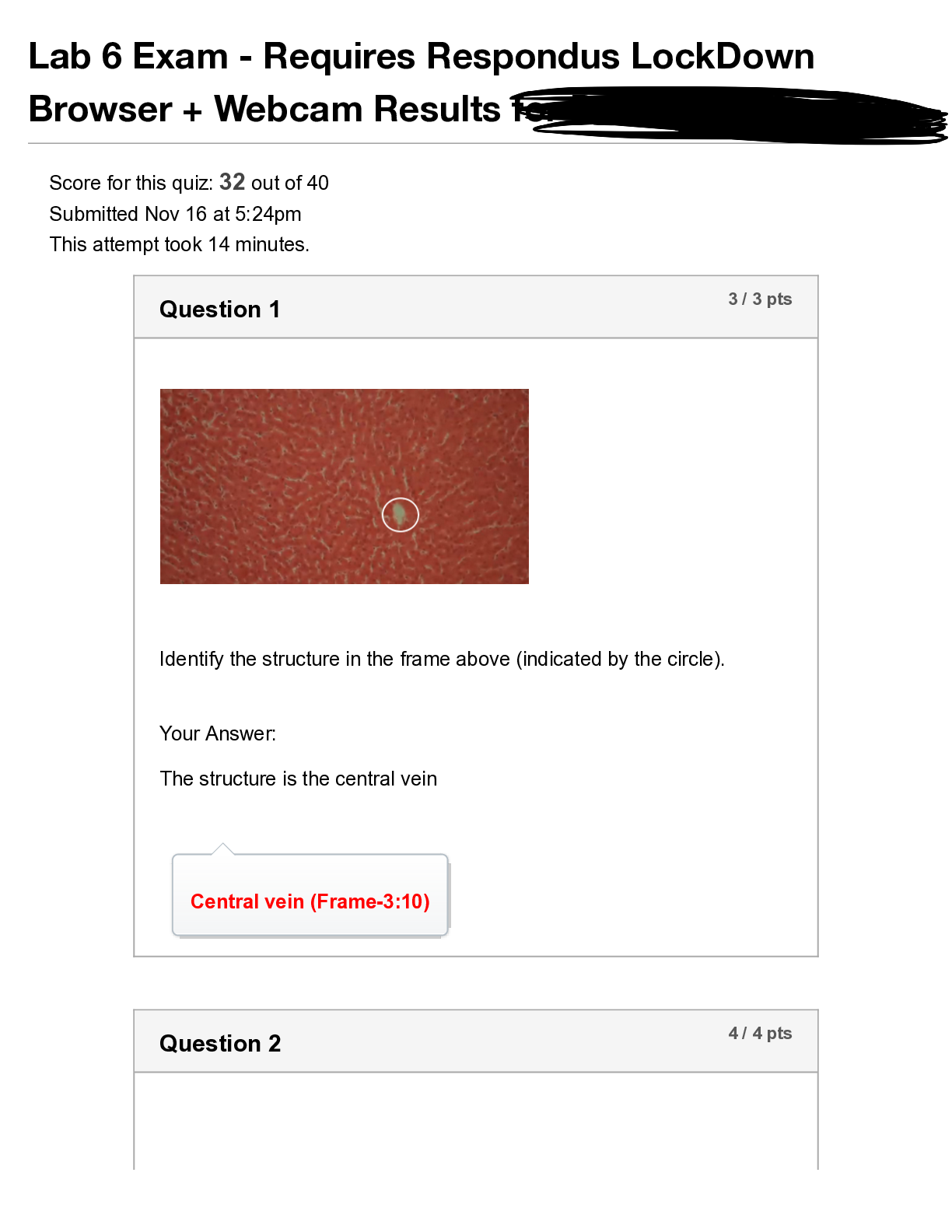
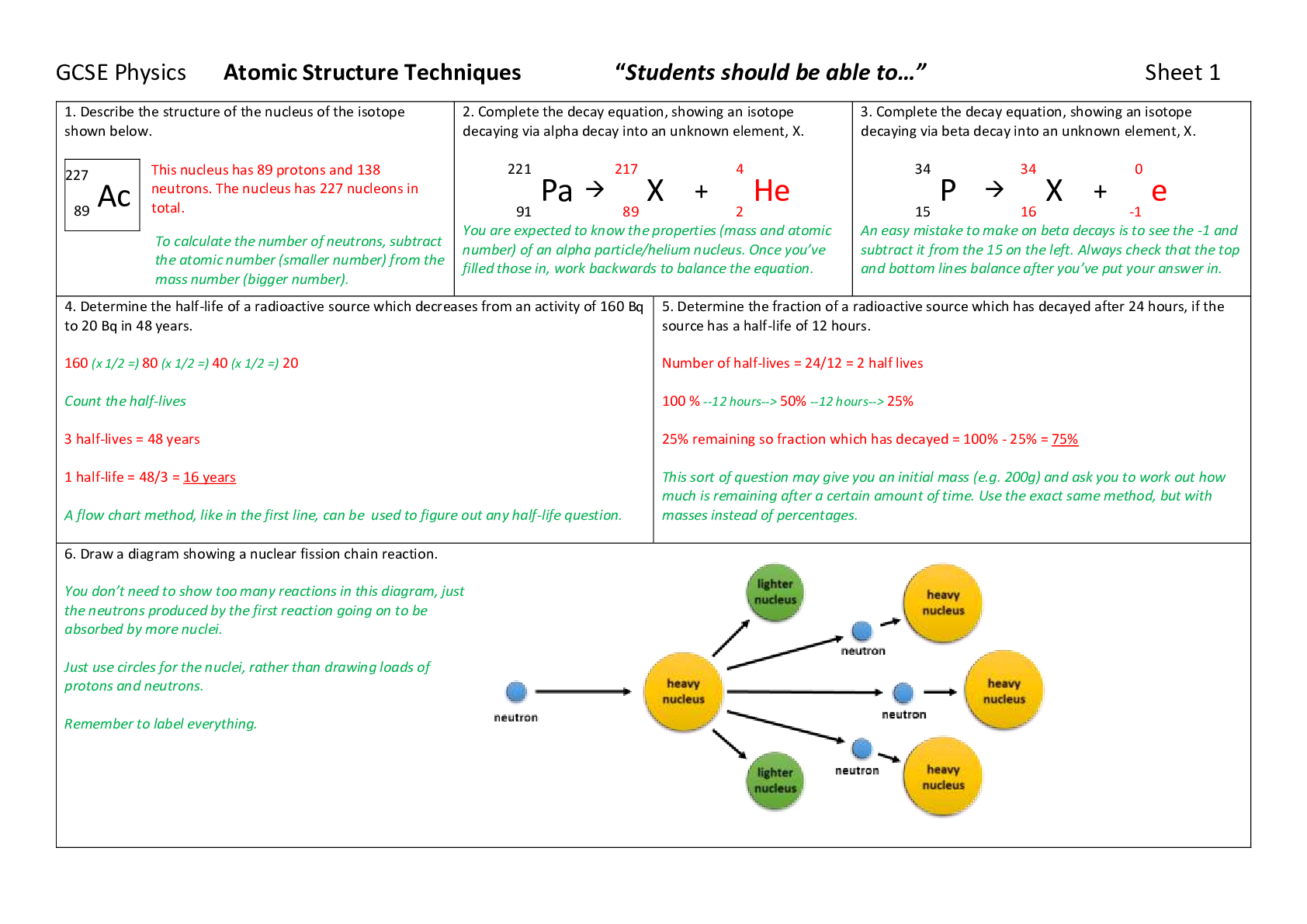
1. Describe the structure of the nucleus of the isotope
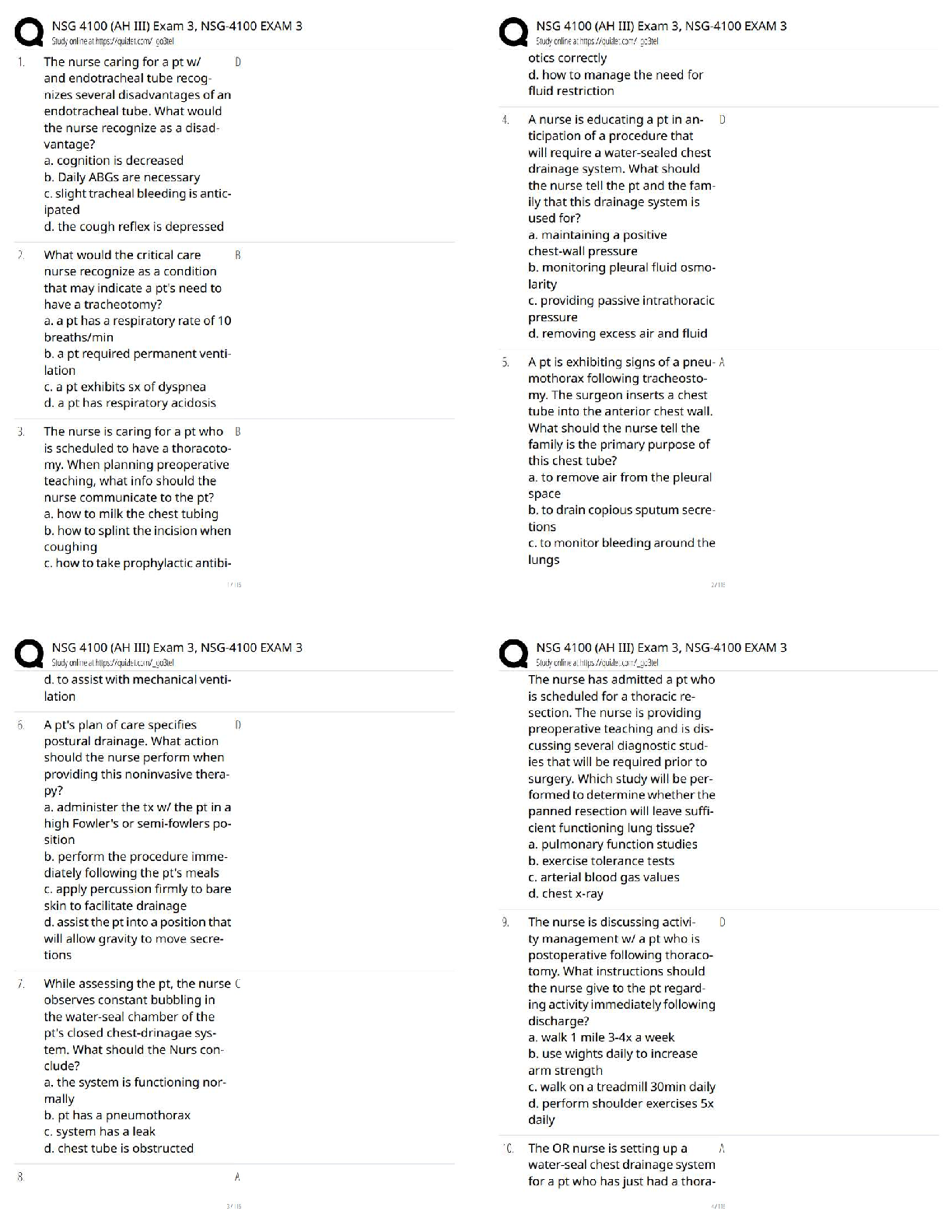
shown below.
227
Ac
This nucleus has 89 protons and 138
neutrons. The nucleus has 227 nucleons in
89 total.
To calculate the number of neutrons, subtract
th
...
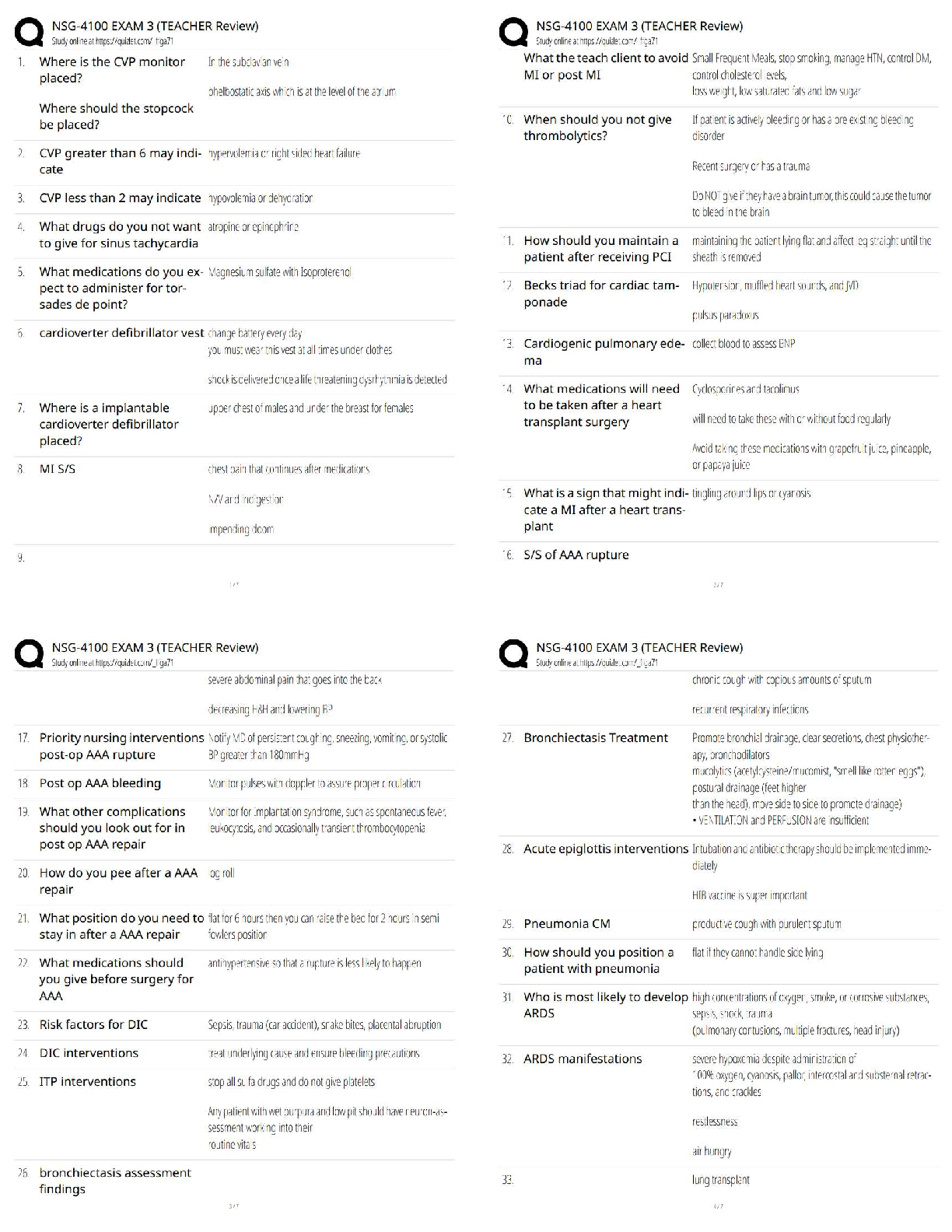
1. Describe the structure of the nucleus of the isotope
shown below.
227
Ac
This nucleus has 89 protons and 138
neutrons. The nucleus has 227 nucleons in
89 total.
To calculate the number of neutrons, subtract
the atomic number (smaller number) from the
mass number (bigger number).
2. Complete the decay equation, showing an isotope
decaying via alpha decay into an unknown element, X.
221
Pa
217
X +
4
He
91 89 2
You are expected to know the properties (mass and atomic
number) of an alpha particle/helium nucleus. Once you’ve
filled those in, work backwards to balance the equation.
3. Complete the decay equation, showing an isotope
decaying via beta decay into an unknown element, X.
34
P
34
X +
0
e
15 16 -1
An easy mistake to make on beta decays is to see the -1 and
subtract it from the 15 on the left. Always check that the top
and bottom lines balance after you’ve put your answer in.
4. Determine the half-life of a radioactive source which decreases from an activity of 160 Bq
to 20 Bq in 48 years.
160 (x 1/2 =) 80 (x 1/2 =) 40 (x 1/2 =) 20
Count the half-lives
3 half-lives = 48 years
1 half-life = 48/3 = 16 years
A flow chart method, like in the first line, can be used to figure out any half-life question.
5. Determine the fraction of a radioactive source which has decayed after 24 hours, if the
source has a half-life of 12 hours.
Number of half-lives = 24/12 = 2 half lives
100 % --12 hours--> 50% --12 hours--> 25%
25% remaining so fraction which has decayed = 100% - 25% = 75%
This sort of question may give you an initial mass (e.g. 200g) and ask you to work out how
much is remaining after a certain amount of time. Use the exact same method, but with
masses instead of percentages.
6. Draw a diagram showing a nuclear fission chain reaction.
You don’t need to show too many reactions in this diagram, just
the neutrons produced by the first reaction going on to be
absorbed by more nuclei.
Just use circles for the nuclei, rather than drawing loads of
protons and neutrons.
Remember to label everything.7. Explain which of the isotopes in the table would be the best choice for use as:
Isotope Half-life Emission (a) a radioactive medical tracer (b) a source in a radiotherapy machine
A 4 hours α C – emits beta and gamma radiation which can
penetrate the body and be detected. Half-life is long
enough for procedure to take place, but not so long that
it will remain the body for a long period of time.
A isn’t suitable as it is a gamma emitter, and won’t
penetrate the body. The half-life of B and E is too long,
and D is too short.
E – Emits gamma radiation which can penetrate all the
way through the body. Half-life is long enough so that it
does not need to be replaced regularly, but short
enough that it is easy to dispose of.
A and B aren’t suitable as alpha/beta wouldn’t
penetrate the body. A, C and D aren’t suitable as half-life
is too short, and would need replacing regularly.
B 2 years β
C 10 days β γ
D 20 minutes γ
E 4 years α β γ
8. Use the graph to accurately determine the half-life of the radioactive source.
Activity /Bq
48
42
36
30
24
18
12
6 0
40 80 120 160 200 240 280 320 360
Time /hours
Half-life = 100 hours
To work this out, go down to half the initial amount and draw a line across to the curve,
then go down to find the corresponding time. Repeat this, to verify that the half-life is
consistent.
9. Describe and explain how the model of the atom has changed over time. You may use
diagrams as part of your answer.
Before the discovery of the electron, atoms were thought to be tiny spheres that could not
be divided. The discovery of the electron led to the plum pudding model of the atom. The
plum pudding model suggested that the atom is a ball of positive charge with negative
electrons embedded in it.
The results from the alpha particle scattering experiment led to the conclusion that the
mass of an atom was concentrated at the centre (nucleus) and that the nucleus was
charged. This nuclear model replaced the plum pudding model. Niels Bohr adapted the
nuclear model by suggesting that electrons orbit the nucleus at specific distances.
Later experiments led to the idea that the nucleus could be divided into smaller, positively
charged particles, which were named protons. The experimental work of James Chadwick
provided the evidence to show the existence of neutrons within the nucleus.
[Show More]