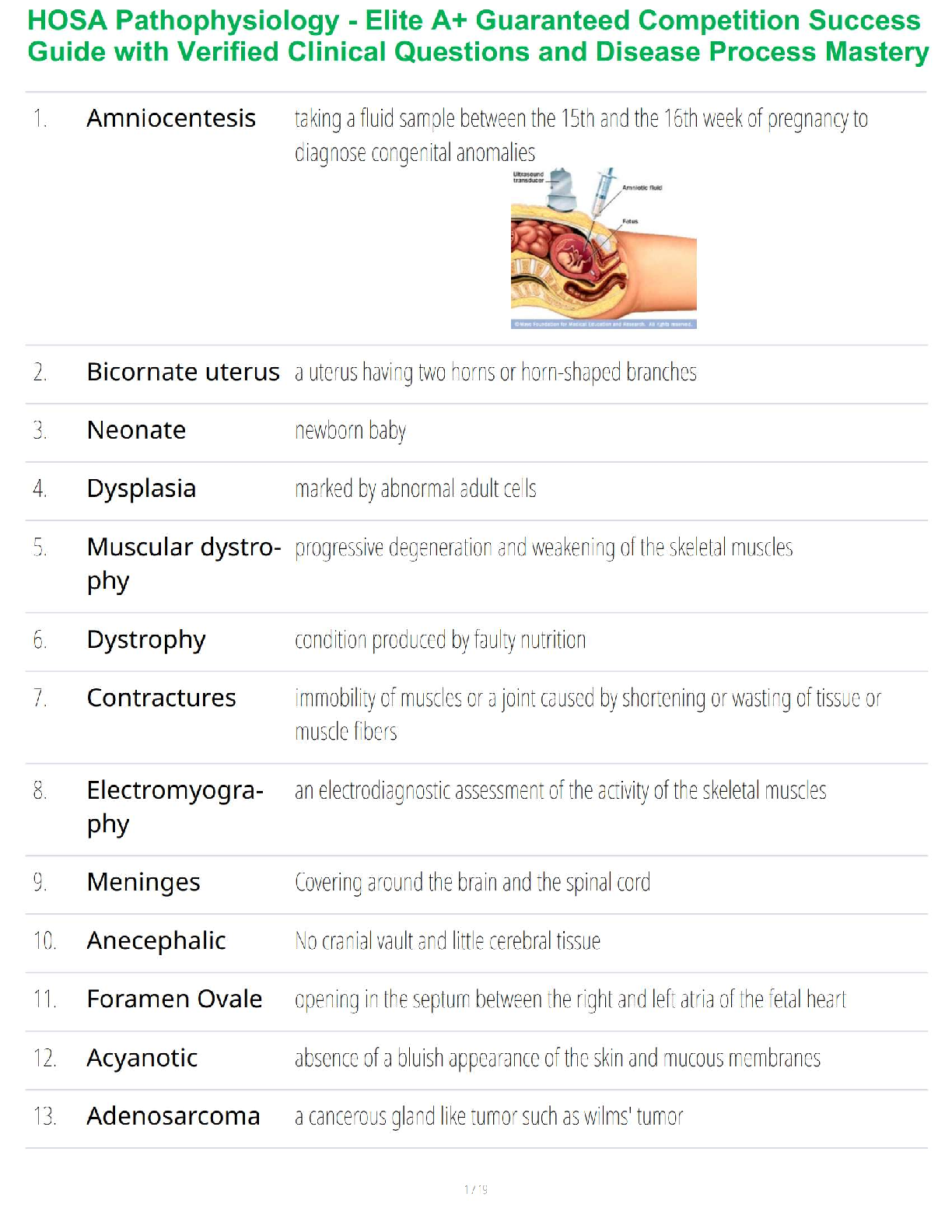
Sterilization and Disinfection
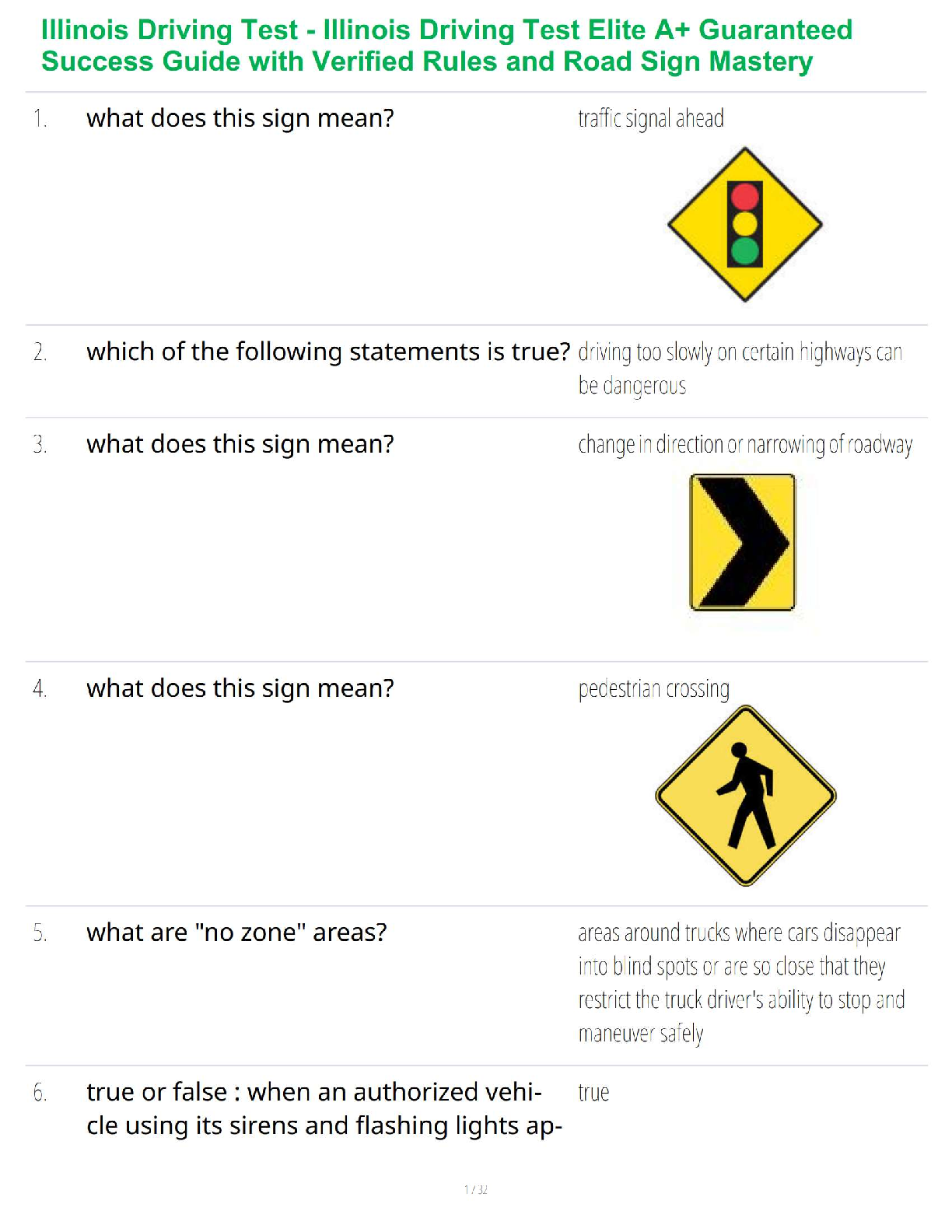
Aseptic technique includes all the methods by which contamination with microorganisms is restricted on the patient, in the environment, equipment and supplies.
Sterile technique (surgi
...
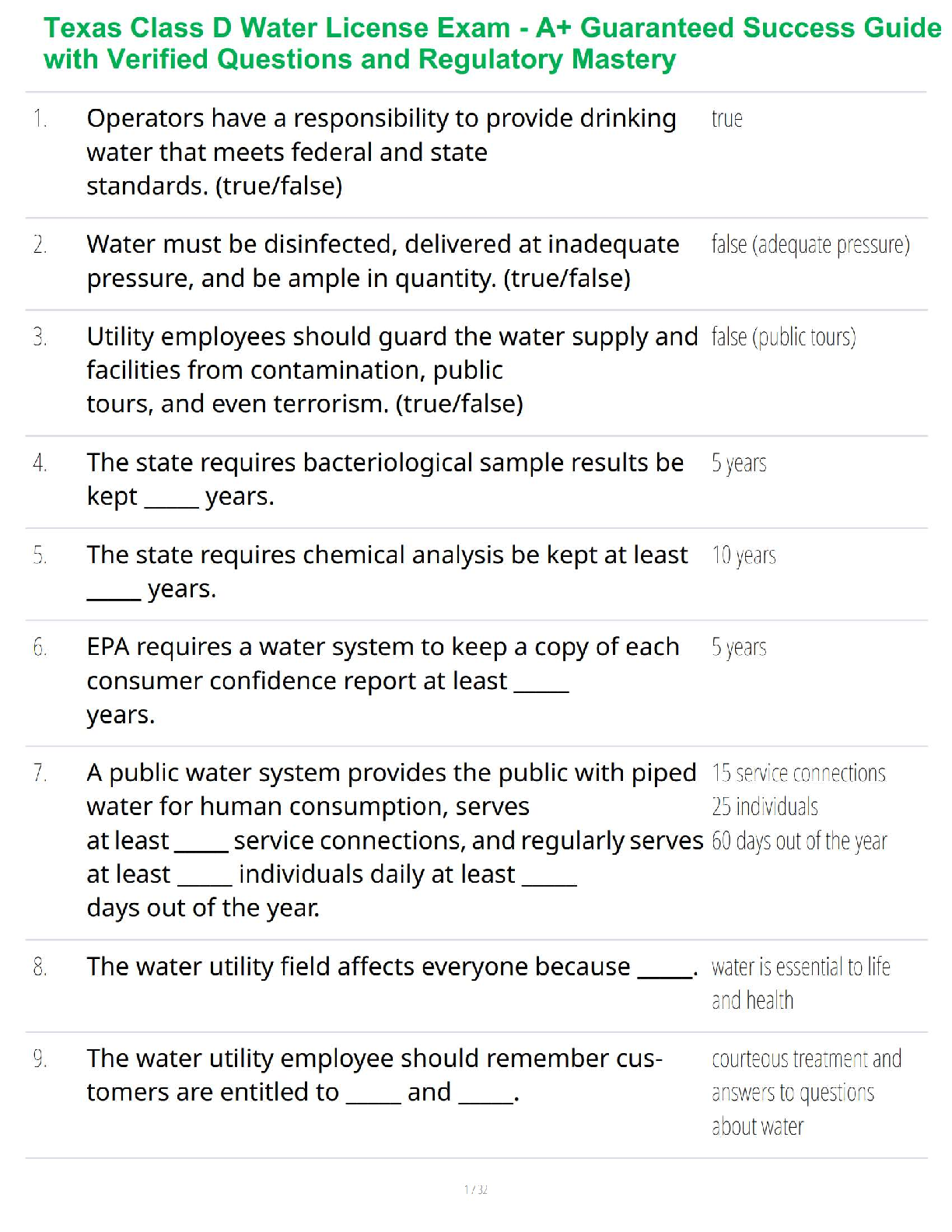
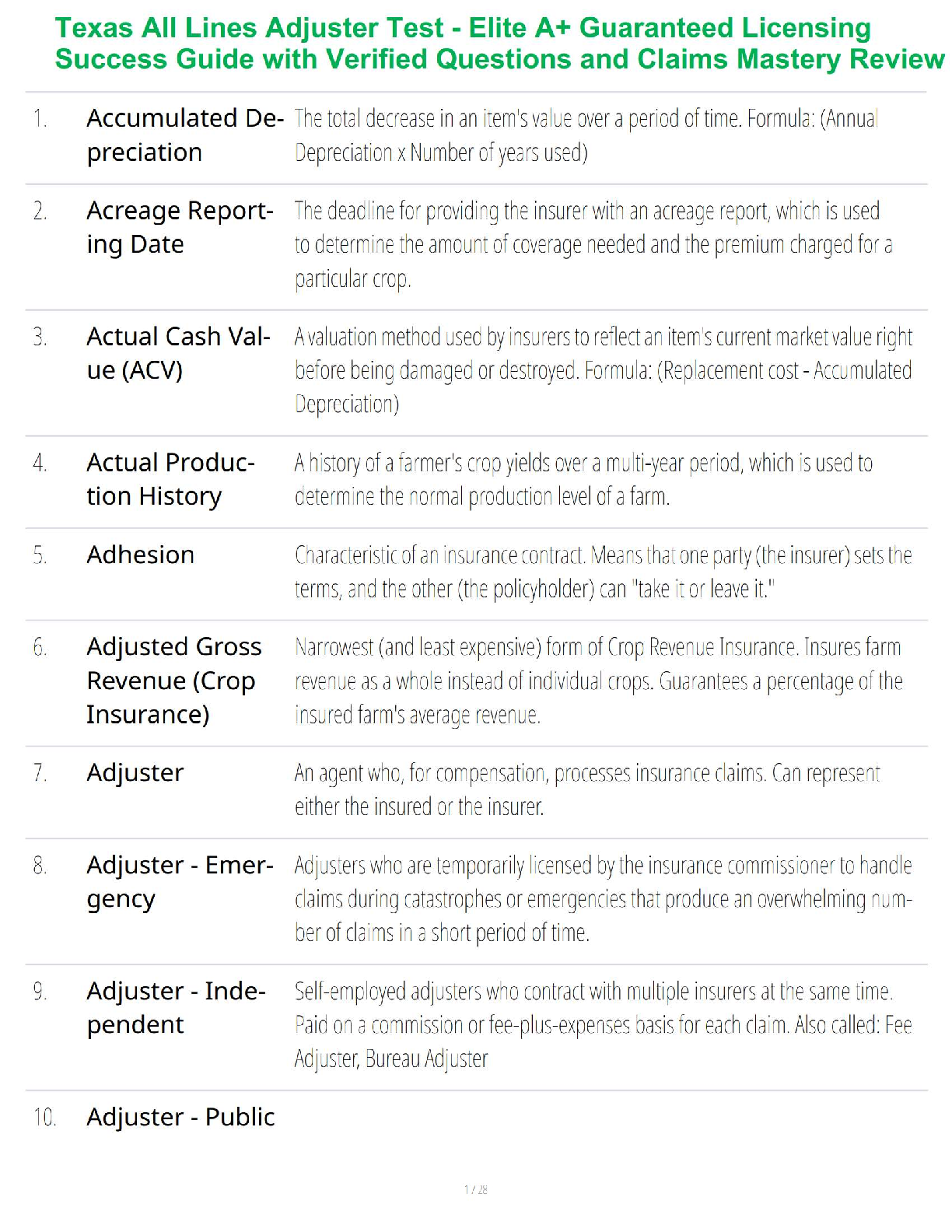
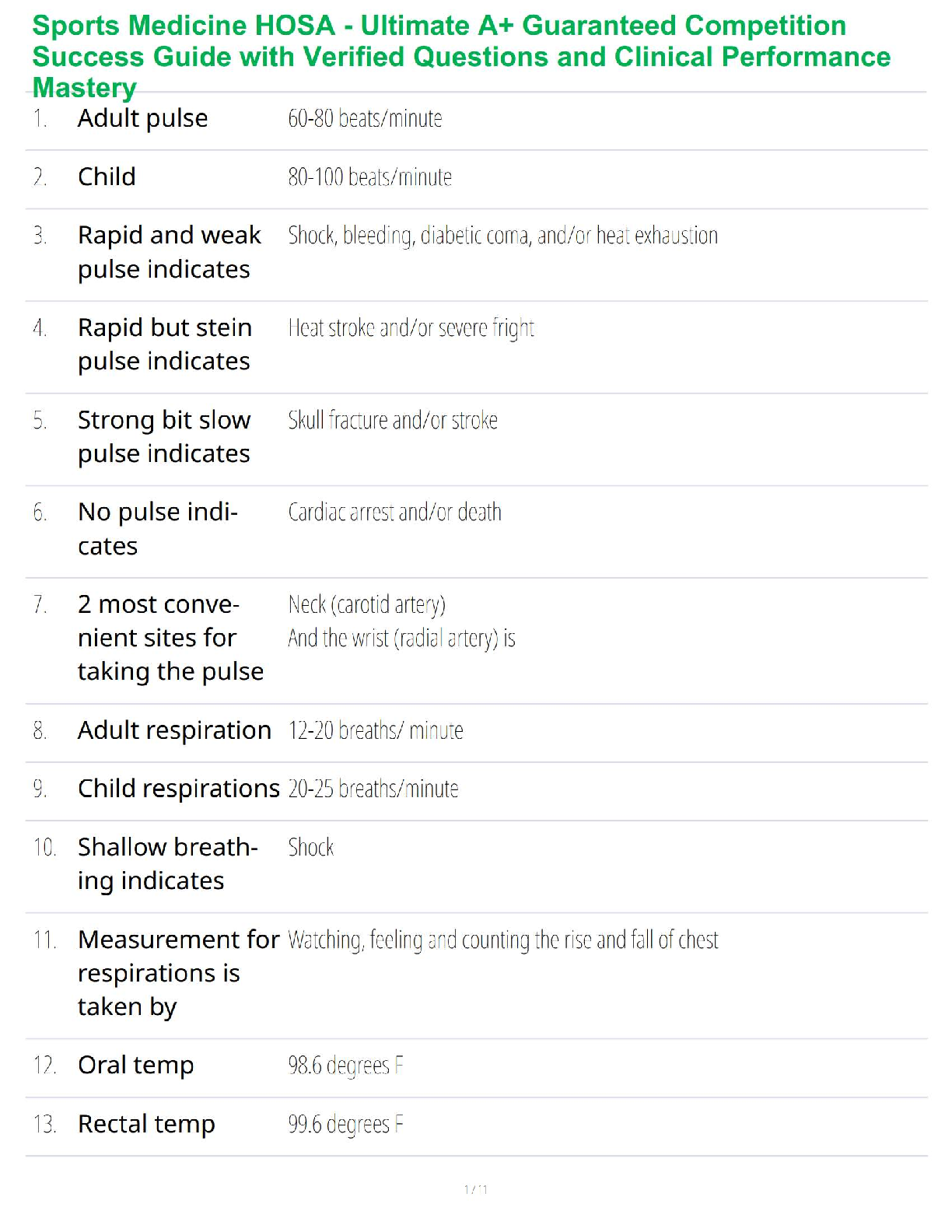
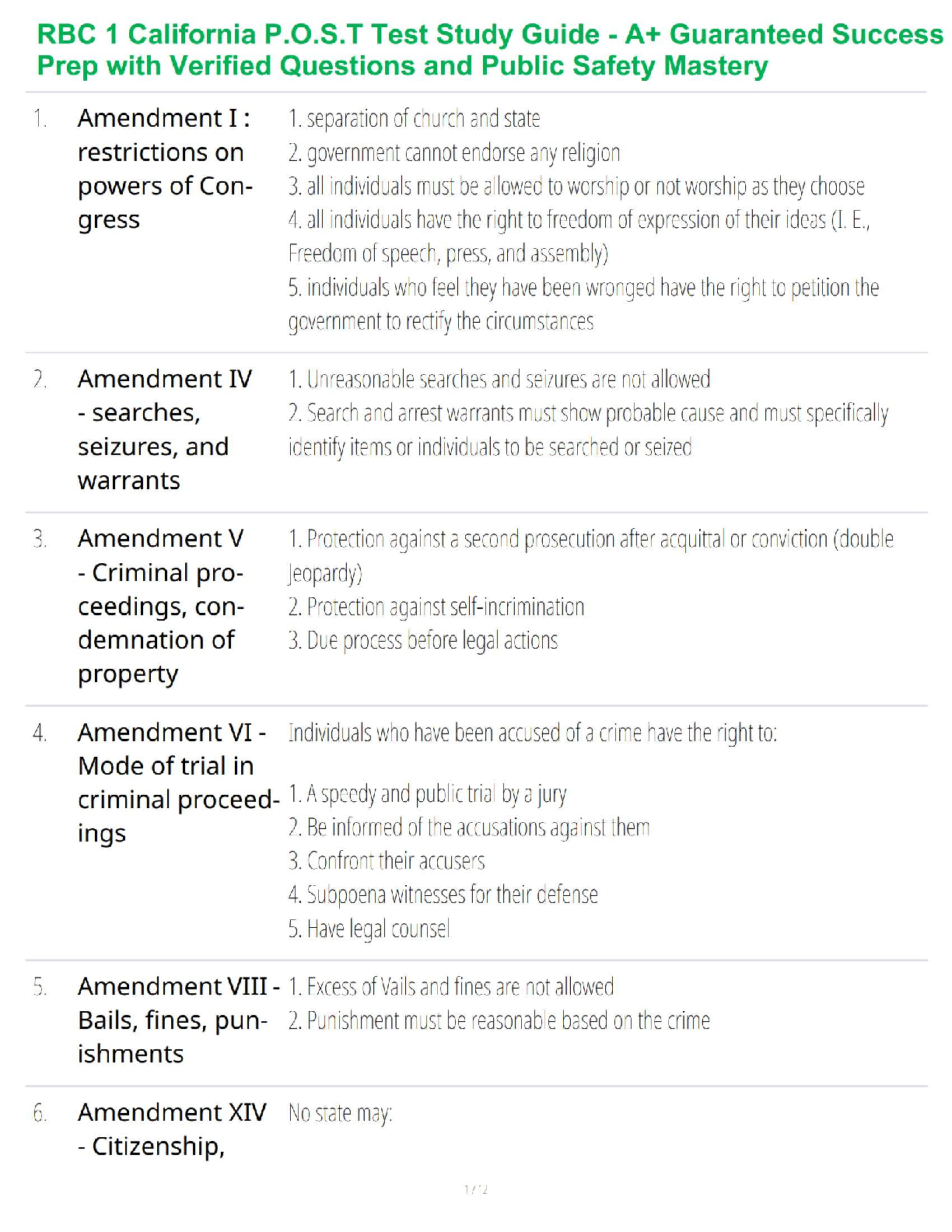
Sterilization and Disinfection
Aseptic technique includes all the methods by which contamination with microorganisms is restricted on the patient, in the environment, equipment and supplies.
Sterile technique (surgical asepsis) comprises methods which are designed to exclude all microbes.
In order to prevent serious complication to the already compromised surgical patient, it is essential that aseptic technique and sterile technique be practiced with absolute accuracy.
Goals of Aseptic and Sterile Technique
The goals of aseptic and sterile technique are to:
Prevent surgical infection
Optimize primary wound healing
Minimize the length of recovery from surgery
Aseptic practices are implemented preoperatively, intraoperatively, and postoperatively to minimize wound contamination. All members of the surgical team must be knowledgeable about aseptic technique and practice strict adherence to aseptic technique principles.
Sterile technique comprises methods by which contamination of an item on the sterile field is prevented by maintaining the sterility of the item/area involved in the procedure.
However, it is the perioperative nurse who monitors the implementation of aseptic techniques by all surgical team members and ensures that breaks in technique are corrected.
Principles of Aseptic Technique
1. All items used within a sterile field must be sterile.
2. A sterile barrier that has been compromised must be considered contaminated.
3. The edges of a sterile wrapper or container are considered unsterile once the package is opened.
4. Gowns are considered sterile in front from shoulder to the level of the surgical field, and the sleeves to two inches above the elbow. The back of the gown is considered unsterile.
5. Tables are sterile at table level only.
6. Sterile persons and items touch only sterile areas; unsterile persons and items touch only unsterile areas.
7. Movement within or around a sterile field must not contaminate the field.
8. All items and areas of doubtful sterility are considered contaminated.
"When in doubt, throw it out."
Recommended Practices for Maintaining a Sterile Field
As a means of preventing infection during any invasive procedure, AORN established the following Recommended Practices for maintaining a sterile field as a guide for anyone who is present during the procedure, either directly or indirectly involved with patient care. These recommendations are based on the knowledge of epidemiology and microbiology and can be applied to any area where invasive procedures are being performed.
Scrubbed persons should function within a sterile field.
Sterile drapes should be used to establish a sterile field.
Items used within a sterile field should be sterile.
All items introduced to a sterile field should be opened, dispensed, and transferred by methods that maintain sterility and integrity.
A sterile field should be maintained and monitored constantly.
All personnel moving within and around a sterile field should do so in a manner that maintains the sterile field.
Policies and procedures for maintaining a sterile field should be written, reviewed annually, and readily available within the practice setting.
Additional Recommendations
In addition to the seven Recommended Practices, additional methods can be used to protect and maintain an aseptic environment.
Contamination of a bacterial barrier should be prevented by eliminating/reducing potential sources of contamination such as air, moisture, or dust.
Talking in the room should be restricted to a minimum.
Unscrubbed personnel should approach sterile fields facing them and never walk between two sterile fields. When moving around the sterile area, maintain a safe distance from the sterile field.
Scrubbed personnel should move from sterile areas to sterile areas. If they must change positions, they should turn back-to-back or face-to-face while maintaining a safe distance between each other.
OR doors should be kept shut except during movement of patients, personnel, supplies, and equipment, and minimize the amount of traffic in and out of the OR during the procedure.
Breaks in technique should be monitored, and if they occur, immediate and appropriate steps should be taken to correct the resulting contamination, for example, changing torn gloves.
Surgical Conscience
“…With loyalty will I endeavor to aid the physician in his work, and devote myself to the welfare of those committed to my care.” The Nightingale Pledge (1893)
Surgical conscience is that concept which allows for no compromise in the principles of aseptic technique, since anything less could increase the potential risk of infection, resulting in harm to the patient.
A surgical conscience builds on the principles of aseptic technique and is an act of mental discipline. It involves the assessment and regulation of one’s own practice, with particular attention to deviations from acceptable, safe practices.
It demands the recognition of improper practices observed during surgery by any member of the health care team, and the ability to report one’s own breaks in technique so that corrective action can be taken. Additionally, it involves the ability to set aside personal preferences and prejudices in order to provide optimum patient care. When fully developed, surgical conscience can become a blend of integrity, honesty, and self-confidence, because it recognizes the human trait of being fallible, requiring alertness to errors, and correcting them before another person is harmed.
Simply stated, surgical conscience is doing unto others as you would have others do unto you. Florence Nightingale once summarized this concept by stating,
“The nurse must keep a high sense of duty in her own mind, must aim at perfection, and must be consistent always in herself.” ------ Florence Nightingale
Microbiology Review
All basic procedures of cleaning, sterilizing, disinfecting, and maintaining the sterility of supplies is based on our knowledge of bacteria. Microorganisms are in the food we eat, the air we breathe, the water we drink, the ground we walk on, and on all things around us including our bodies. Not all microorganisms are normally pathogenic or capable of causing disease. In fact, with most microorganisms, humankind generally enjoys a state of peaceful coexistence. The patient is a source of endogenous infection because of the large number of microorganisms found in and on the body.
Risk Factors Which Contribute to Postoperative Infections
The skin is the first line of defense against the entry of microorganisms into the body. When the skin is incised, the patient is immediately exposed to the risk of infection.
In addition, certain factors or conditions, if present, can significantly affect a patient’s risk for developing a postoperative wound infection.
Pre-existing infection
The presence of infection anywhere in the body is always a contraindication for elective surgery.
Surgical technique
Although necessary, catheters and drains may increase the risk of infection, because they provide a pathway for the migration of organisms.
Length/type of surgery
The risk of infection increases with the length of exposure of internal tissues to the environment, the presence of implants, and the amount of ischemic tissue present.
Pre-existing diseases and conditions
Traumatic wounds and surgical procedures that require entering the respiratory and gastrointestinal tracts increase the risk of infection because large numbers of organisms are ordinarily found in these areas.
Impaired defense mechanisms
Defense mechanisms are impaired in the immunosuppressed, for example, those patients receiving radiation, corticosteroids, or chemotherapy. Additional stress is placed on the defense mechanisms of patients with chronic conditions such as diabetes, alcoholism, cancer, and cardiac and respiratory diseases.
Nutritional status
Malnutrition lowers the body’s ability to fight the infectious process. Obesity is a risk factor because less blood is supplied to fatty tissue, and lower tissue perfusion increases the susceptibility to infection.
Age
Premature and newborn infants and geriatric patients share an increased risk of developing an infection.
Impaired skin integrity
Impaired skin integrity increases the risk of infection.
Classification of Surgical Wounds
The Centers for Disease Control and Prevention (CDC) has established a classification system for surgical wounds.
The purpose of this classification is:
To facilitate tracking infections involving the surgical patient
To learn how they may have occurred
To learn how to prevent their recurrence
The system is based on risk factors for postoperative infections based on characteristics of the surgical procedure.
The system defines four categories of surgical wounds:
Category Type of wound
Class I Clean
Class II Clean contaminated
Class III Contaminated
Class IV Dirty or infected
Placing a surgical procedure in its proper category is an important responsibility of the perioperative nurse, because it will help the infection control department assess trends in postoperative infections and develop action plans to reduce these infections.
Class I - Clean Wounds
Clean wounds are clean operative wounds in which:
No inflammation is encountered
The respiratory, alimentary, and genitourinary tracts are not entered
Clean wounds are usually elective surgery with primary closure and may have a closed drainage system.
What is the percentage of risk for developing a postoperative infection due to a clean wound?
With this class, there is a 1% to 5% risk of postoperative infection.
Examples of clean wounds include (but are not limited to):
Eye surgery
Hernia repairs
Breast surgery
Non-traumatic neurosurgery
Non-traumatic orthopedic, cardiac, or peripheral vascular surgeries
Class II - Clean Contaminated Wounds
What is a clean contaminated wound? Clean contaminated wounds are operative wounds in which:
The respiratory, alimentary, or genitourinary tract is entered under controlled conditions and without contamination of the surrounding tissue
There is no evidence of infection or no major break in aseptic technique
What is the percentage of risk for developing a postoperative infection due to a clean contaminated wound?
With this class, there is a 8% to 11% risk of postoperative infection.
What are the examples of clean contaminated wounds?
Examples include (but are not limited to):
Dilation and curettage (D&C)
Total abdominal hysterectomy (TAH)
Gastrectomy
Cholecystectomy without spillage
Elective appendectomy
Cystoscopy (cysto)
Transurethral resection (TUR) bladder procedures on a patient with negative urine cultures
III - Contaminated Wounds
What is a contaminated wound?
Surgery that involves:
Open, fresh, traumatic wounds
Major breaks in sterile technique
Gross spillage from the GI tract
Incisions in which acute, nonpurulent inflammation is encountered
What is the percentage of risk for developing a postoperative infection due to contaminated wounds?
There is a 10% to 17% risk of postoperative infection.
What are the examples of contaminated wounds? Examples of contaminated wounds include (but are not limited to):
Rectal surgery
Laparotomy with significant spillage
Traumatic wounds, for example, gunshot, stab wounds without perforation of viscera
Acute inflammation of any organ without frank pus present, for example, acute appendicitis or cholecystitis, compound fractures
Class IV - Dirty or Infected Wounds
What is a dirty wound? Dirty or infected wounds include old traumatic wounds with:
Retained devitalized tissue
Wounds that involve existing clinical infection or perforated viscera and/or delayed primary closure of wounds
This classification suggests an infectious process was present before surgery.
What is the percentage of risk for developing a postoperative infection?
There is a greater than 27% risk of postoperative infection.
What are the examples of dirty wounds? Examples of dirty wounds include (but are not limited to):
Incision and drainage
Total evisceration
Perforated viscera
Amputations
Decontamination, Disinfection, and Sterilization
Attempts to control equipment-related sources of infection include decontamination, sterilization, and disinfection. All supplies and instruments that are intended for use during the surgical procedure must have been decontaminated and sterilized or disinfected prior to patient use. Although many tasks involved in instrument cleaning and sterilization are assigned to ancillary personnel, it is important that perioperative nurses have complete understanding of the processes involved because they share accountability for determining whether an item is safe for use.
Processes Necessary for Sterilization
Instruments, supplies, and other equipment must be readied for use according to acceptable practices through a group of processes. It is important that all personnel know how to execute these tasks correctly and safely and be able to distinguish the processes that achieve sterility from those that merely provide high-level disinfection. The processes necessary for sterilization are as given below.
1. Decontamination
2. Cleaning
3. Assembly
4. Packaging
5. Sterilization Processes in Sterilization
6. Storage
7. Transportation
Decontamination
Decontamination is defined as any physical or chemical process that serves to reduce the number of microorganisms on any inanimate object to render that object safe for subsequent handling.
Decontamination is the first step toward reducing the potential hazards associated with direct contact with blood, fluids, or tissues on contaminated instruments. Decontamination refers to a process in which the bioburden is reduced and contaminants are removed, either by hand cleaning or mechanical methods. Specific solutions and equipment are used which remove the blood and debris from the surface of an object or instrument, thereby making them safe to handle.
Once rendered safe for handling through a decontamination process, patient care items should undergo a process of either disinfection or sterilization.
AORN's Recommended Practices for Sterilization in Perioperative Practice Setting includes the following Recommended Practice.
Recommendation I - Items to be sterilized should be cleaned and decontaminated in a controlled environment and in accordance with a device manufacturer's written instructions.
AORN's Recommended Practices for Cleaning and Caring for Surgical Instruments and Powered Equipment includes the following Recommended Practices.
Recommendation IV - Instruments should be kept free of gross soil during surgical procedures.
Recommendation V - Cleaning and decontamination should occur as soon as possible after instruments and equipment are used.
Recommendation I
Recommended Practices for Cleaning and Caring for Surgical Instruments and Powered Equipment - Recommendation I states:
"The manufacturer’s written, validated instructions for handling and reprocessing should be obtained and evaluated to determine the ability to adequately clean and reprocess the equipment within the health care facility before purchasing surgical instruments and powered equipment."
Surgical instruments and powered equipment should be cleaned, handled, and used according to the manufacturers' instructions.
Cleaning and handling instructions recommended by the device manufacturer vary widely. Specific types of equipment, pneumatically powered instruments, and specialty instruments can require special cleaning and maintenance procedures.
Recommendation IV
Recommended Practices for Cleaning and Caring for Surgical Instruments and Powered Equipment - Recommended Practice II states:
"Instruments should be kept free of gross soil during surgical procedures."
This RP stipulates the following guidelines:
Cleaning and decontamination should begin the moment an instrument is used in the OR, with the scrub person wiping the gross soil from the instrument with a sponge moistened with sterile water.
Blood and body fluids can cause pitting of instruments and if left to dry, can be difficult to remove.
Instruments with lumens should be kept patent by irrigating with sterile water.
Recommendation V
Recommended Practices for Cleaning and Caring for Surgical Instruments and Powered Equipment - Recommendation V states:
"Cleaning and decontamination should occur as soon as possible after instruments and equipment are used."
This RP stipulates the following guidelines:
Initial decontamination of instruments should begin immediately after the completion of any invasive procedure to prevent the formation of biofilm. For example, the instruments may be placed in a specially designed container for presoaking instruments or covered with a water moistened towel and placed in a plastic bag.
Never soak stainless steel or other metal devices in saline. The chloride ions in saline may cause corrosion of the metal.
Cleaning and decontamination must be thoroughly accomplished or disinfection and sterilization may not be effective
Objectives of Decontamination
Cleaning is the single most important step in making a medical device ready for reuse. Debris left on a device can interfere with contact with the sterilant, affect its function, or lead to a foreign body reaction in a patient if introduced into the body. There are four objectives in the cleaning process:
Removal of visible soil which includes blood, tissue, and bone
Removal of invisible soil such as microorganisms
Preparation of the surface of any item for sterilization or disinfection in order to allow direct contact with the sterilizing or disinfecting agent
Protection and safety of patients and personnel
Basic Principles of Decontamination
Decontamination is the first critical step in the sterilization process. Sterilization cannot be achieved if items are not thoroughly cleaned. It is essential to remove any soil on an item before sterilization. It is important to remember that “cooked on” dirt is not sterile dirt. Any soil left on an item being sterilized will act as a barrier between the item and the sterilant. The primary debris/soil is proteinaceous in nature. Coagulated protein may cover and protect bacteria from coming in contact with the sterilizing agent.
Decontamination can be accomplished either by hand or automated mechanical methods. Regardless of the method chosen, some basic principles hold true.
Disassembly
Open box locks and disassemble instruments with multiple parts. It is important to remember that regardless of the method chosen to clean the instruments, all items with detachable parts that can be separated need to be disassembled for cleaning, packaging, and sterilizing.
Enzymatic wash
An enzyme detergent may be helpful in removing debris on items heavily soiled with protein or fat, or if the soil is dried on.
Cool water rinse
When cleaning manually, the next step should be a cool-water rinse to remove gross debris, preventing the possibility of bioburden and/or contaminants from “baking” onto the item.
Appropriate detergent
Instruments with lumens should be flushed with water or detergent solution. Always choose a detergent that is compatible with the materials in the device and suited for the type of soil. The device manufacturer should be consulted for recommendations for the type of detergent to be used.
It is important that personal protective attire be worn by personnel when decontaminating instruments!
Cleaning
Automated cleaning can be performed with one of several types of machines.
Ultrasonic unit
Washer decontaminator sometimes referred to as a disinfector
Washer/sterilizer
AORN has specified the following Recommendation which provides guidelines for cleaning and caring for surgical instruments and powered equipment.
Recommendation I - Items to be sterilized should be cleaned, decontaminated, sterilized, and stored in a controlled environment and in accordance with AORN’s “Recommended practices for cleaning and caring of instruments and powered equipment” and the device manufacturer’s written instructions.
Automated Cleaning Machines
There are several types of machines which perform mechanical cleaning.
Ultrasonic cleaner remove the fine debris from instruments through a process called cavitation. Ultrasonic energy is passed through a water bath, creating bubbles that implode. The process of implosion creates a suction action that pulls debris away from instrument surfaces. The instruments must be rinsed free of gross debris before being placed in the ultrasonic cleaner, or else the sound energy will be absorbed by the larger clumps, making the process ineffective.
Washer decontaminators/disinfectors can be single or multi-chamber tunnel units. The cycles in each type or model differ, but generally include a pre-rinse, an enzymatic soak, a wash with detergent, an ultrasonic cleaning, a sustained hot water rinse, a deionized water final rinse, a liquid chemical germicide rinse, and a drying cycle. Some units include a lubrication cycle. Upon completion of the programmed cycle, the instruments are rendered safe for handling. The chief advantage of this type of unit is that it allows hands-free processing.
Washer/sterilizers have been around for more than 30 years. They process instruments through several cycles, including cold water pre- rinse, high-temperature wash with alkaline detergent, neutralizing cycle, final rinse, and sterilization. The instruments are not considered prepared for subsequent use. The washer sterilizer must have a cold cycle. If heat is used during the process, gross bioburden must be removed or it will bake onto the item.
Recommendation XI
Recommended Practices for Cleaning and Caring for Surgical Instruments and Powered Equipment states:
"Surgical instruments should be inspected for cleanliness and proper working order after decontamination."
This RP stipulates the following guidelines:
Inspecting instruments before assembly of trays provides an opportunity to identify those instruments that require additional cleaning or repair before use.
Unless otherwise specified, lubricants should be water-soluble in order to allow steam penetration during sterilization. Oil-based lubricants cannot be penetrated by steam.
Always follow the manufacturers’ instructions.
Recommendation XVII
Recommended Practices for Cleaning and Caring for Surgical Instruments and Powered Equipment states:
"Special precautions should be taken to minimize the risk of transmission of prion diseases."
Creutzfeldt-Jakob disease (CJD) is a degenerative neurologic disease that is caused by agents commonly known as “unconventional viruses”, that is, prions. Prions are not bacteria or viruses but rather malformed proteins. Prions are resistant to most forms of sterilization used in health care settings and have a marked resistance to being inactivated by moist heat.
There is no known or suspected risk of transmission to health care workers handling CJD-contaminated items when wearing proper PPE and having intact skin.
Information about prions, prion infectivity, and decontamination is evolving. The Centers for Disease Control and Prevention and the World Health Organization, as well as material published by experts in the field, provide updates. AORN’s Recommended Practices for Cleaning and Caring for Surgical Instruments and Powered Equipment goes into further detail, describing general measures for cleaning instruments and the environment when prion disease is known.
Assembly
The next step in preparing items for sterilization is assembling the instruments. During the assembly process, instruments should be inspected for:
Cleanliness
Proper function and alignment
Corrosion, pitting, burrs, nicks, and cracks
Sharpness of cutting edges
Looseness of set pins
Wear and chipping of inserts and plated surfaces
Any other defects
It is necessary to follow a few guidelines during the assembly process.
Instruments in disrepair should be removed from service until properly repaired or replaced.
Instruments should be arranged in sets and placed in a tray with a mesh or perforated bottom or specifically designed container that permits the penetration of the sterilizing agent and prevents the trapping of air. A towel or other absorbent material may be placed in the bottom of the tray to help absorb condensate that is formed during sterilization and help speed the drying process. Towels can be added to a tray if the items are to be sterilized using steam or ethylene oxide.
All instrument joints and hinges must be opened to ensure contact with the sterilizing agent. All instruments with detachable parts must be left disassembled.
Peel pouches should not be used to protect delicate/sharp instruments inside sterilization trays. The sterilization agent may not be able to penetrate the pouch
Steam Sterilization
During steam sterilization, an absorbent towel or other porous material must separate items such as basins and medicine cups that are nested. This allows for steam contact with all surfaces. Sterilization can be achieved only if the sterilizing agent can contact all surfaces.
Packaging
The next step in preparing items for sterilization includes packaging. There are a few features which a packaging system should possess.
Permit sterilization to take place.
Maintain sterility until the package is opened or the integrity of the package is compromised.
Provide for aseptic delivery of the contents onto the sterile field.
Be compatible with the sterilization process.
Be used according to the manufacturer’s written instructions.
Be labeled according to the policies and procedures of the practice setting and according to manufacturer’s instructions.
Packaging materials include woven fabrics, non-woven materials, peel pouches of plastic and/or paper, and rigid container systems. Paper/plastic pouches can be used with steam sterilization, but polyethylene pouches are needed for ethylene oxide, hydrogen peroxide gas plasma, and ozone. If the sterility of an item is to be considered event-related, the packaging material should be strong enough to maintain its integrity after the sterilization process and to protect the item from contamination.
Sterilization
Sterilization is defined as the process by which all forms of microbial life—including bacteria, viruses, spores, and fungi—are destroyed to an acceptable sterility assurance level. An item is considered sterile when all parameters of sterilization have been met.
Various types of sterilizations techniques are in use such as:
Saturated steam sterilization
Immediate-Use Steam Sterilization
Chemical sterilization
Ethylene oxide sterilization
Low-temperature hydrogen peroxide gas plasma sterilization
Ozone sterilization
Saturated Steam Sterilization
Steam, at atmospheric pressure, is incapable of sterilizing an item. When steam is placed under pressure, its temperature rises, and the moist heat produced destroys the protein within the cell, rendering it harmless. Direct contact with the steam is required to ensure sterility. Saturated steam under pressure is still the most common and economical method of sterilization for heat-tolerant items used in the OR. It is easily supplied and controlled. Steam does not leave any toxic residue and remains the fastest method of sterilization. However, heat-sensitive items cannot be steam sterilized. Two basic types of steam sterilizers are used:
Gravity displacement sterilizer
Gravity displacement sterilizers rely on gravity to remove air, which is heavier than steam. Air and steam do not mix well; therefore, a layer of steam forms above the air. As more steam is introduced, the space in the chamber decreases and the air exits down the sterilizer's drain.
Dynamic air removal sterilizer
Dynamic air removal sterilizers are similar to gravity displacement sterilizers, except they are fitted with a vacuum pump to ensure that air is removed from the sterilizing chamber and from the load before the steam is injected. A dynamic air removal sterilizer is considered more efficient in removing air than the gravity displacement sterilizer.
Parameters Required for Saturated Steam Sterilization
There are four parameters that affect the steam sterilization process.
Steam saturation
Pressure – 16 to 35 psi
Temperature – 250º to 275º F. (121º to 135º C.)
Time – Temperature is maintained for a prescribed amount of time to achieve the elimination of microorganisms
A few guidelines need to be followed during saturated steam sterilization.
In general, exposure time required to sterilize wrapped items is 30 minutes at 250º F. (121º C.) or 15 minutes at 270º F (132º C) in a gravity displacement sterilizer.
In a dynamic air removal sterilizer 4 minutes at 270º F. (132º C.) or 3 minutes at 275º (135 º C).
Saturated steam sterilized items must be allowed to cool thoroughly before handling or removing from the sterilizer cart.
Items might still contain steam vapor and condensation. If handled, there is a potential for compromising the barrier properties of the packaging materials. The vapor that may remain in these packages might be sufficient for microorganisms from your hands to pass through the material of the wrapper or pouch.
Woven and non-woven wrappers and peel pouches are barriers to contamination only when they are dry.
With rigid containers, the potential for contamination depends on the design of the container.
If a warm, humid item is placed on a cool, unsterile surface, the vapor that remains in the package can condense into water. If this water drains to the outside of the package, strike-through may occur causing the package to be contaminated.
Items should not be moved or touched until they have cooled down to room temperature, usually 30 minutes to 2 hours. During this time, they should be left on a removable cart that has been pulled out of the chamber and placed in a suitable location.
Sterilized items must be cooled down in an area free of traffic and strong air currents
Immediate-Use Steam Sterilization
Immediate-use steam sterilization implies speed…it does not mean that you can take shortcuts! Click the 'Know More' icon to learn more about sterilization of implants. Immediate-use steam sterilization is a process designed for the steam sterilization of patient care items for immediate-use. Immediate-use steam sterilization should be used only when there is insufficient time to process by the preferred wrapped or container method. Immediate-use steam sterilization should not be used as a substitute for adequate instrument inventory.
The exposure phase for immediate-use steam sterilization has as much lethality as the regular steam sterilization cycle. Problems arise if items are not properly cleaned and decontaminated before sterilization or when they are contaminated along the route from the sterilizer to the sterile field. Failure to correctly perform every step in the process leads to failure of the entire process.
Immediate-use steam sterilization should be performed only if all of the following conditions are met:
The device manufacturer’s written instructions on cycle type, exposure times, temperature settings, and drying times (if recommended) are available and followed.
Items are disassembled and thoroughly cleaned with detergent and water to remove soil, blood, body fats, and other substance
Lumens are brushed and flushed under water with a cleaning solution and rinsed thoroughly.
Items are placed in a closed sterilization container or tray, validated for immediate-use steam sterilization, in a manner that allows steam to contact all instrument surfaces.
Measures are taken to prevent contamination during transfer to the sterile field. Immediate-use steam sterilized items are to be used immediately and not stored for later use.
Sterilization of Implants
Implants should not be immediate-use steam sterilized because of possible patient complications. Careful planning, appropriate packaging, and inventory management can eliminate the need to immediate-use steam sterilize implantable medical devices.
Peracetic Acid Sterilization
Peracetic acid can only be used for surgical items that can be immersed. Manufacturer's written directions must be followed regarding maintenance of peracetic sterilizers including filter changing.
Peracetic acid sterilization has the following features:
A chemical sterilization unit that uses a buffered solution of peracetic acid.
The unit automatically dilutes the peracetic acid to a concentration of approximately 2000 ppm.
The items to be processed are exposed to the solution for 12 minutes at 122º to 131.9º F (50º to 55º C.).
The proper selection and use of adaptors to ensure contact of the sterilant to all internal lumens must be verified by health care personnel.
Afterwards, the processed items are rinsed 4 times with sterile water provided by tap water that is passed through a membrane filter. (This filter meets the United States Pharmacopoeia criteria for sterilization of pharmaceuticals by membrane filtration.)
The total processing time is 25 to 30 minutes.
An internal self-diagnostic and monitoring system produces a printed document confirming that all sterilization parameters have been met during each cycle. Peracetic acid is a liquid chemical sterilization method that uses low temperatures of 122º to 131.9º F. (50º to 55.5º C.) and is equal in efficacy to steam and ethylene oxide (EO) sterilization. It requires the use of a special unit designed for this type of sterilization.
Advantages of Peracetic Acid Sterilization
Rapid cycle time of the processor
Low temperatures for heat-sensitive instruments
Environmentally friendly products (peracetic acid, oxygen, water)
Absence of adverse health effects to the operators
Compatibility with wide variety of materials and instruments
Limitations of Peracetic Acid Sterilization
Peracetic acid sterilization also has the following limitations:
Potential material incompatibility, for example, aluminum anodized coatings become dull
Unsuited for moisture-sensitive instruments
Occasional noxious odor similar to vinegar
Items must fit within one of the processing trays and containers provided, which limits the number of items that can be processed at one time
Processed items cannot be stored for use at a later time; they must be used immediately
Ethylene Oxide Sterilization
Ethylene oxide (EO) is a colorless, flammable, explosive gas that is commonly used to sterilize heat and moisture sensitive items. It is a hazardous agent and toxic carcinogen and mutagen that is extremely hazardous when inhaled or when it comes in contact with the eyes or skin. Stringent safety precautions must be followed when using the EO sterilization process.
EO has been proven to be an effective sterilization method due to its ability to penetrate packaging materials, device lumens, and medical devices with complex geometry. It is effective against virtually all microorganisms including bacterial spores, molds, yeasts, and viruses. Achieving complete sterilization in EO is dependent upon four primary variables:
Proper exposure time – 2 to 10 hours
Temperature – 100° to 140° F (37.8° to 63° C)
Chamber humidity of 45 to 75%
Use of the gas concentration required by the system being used
Failure of any one of these variables affects the probability of achieving sterilization.
EO and CFCs
In the past, EO was mixed with chlorofluorocarbons (CFCs) to reduce fire and explosion hazards. Because of concerns about the effect of CFCs on the ozone layer, they are no longer being produced for general use. New technologies using 100% EO, EO/carbon dioxide, or EO/Hydrochlorofluorocarbon (HCFC) mixtures are available
Limitations of Ethylene Oxide Sterilization
Ethylene oxide sterilization has a few limitations.
Items sterilized using EO must be aerated for the prescribed amount of time (12-16 hours) to protect employees and patients.
EO residue cannot be removed by rinsing in water or other liquids.
When EO mixes with water, ethylene glycol is formed which is a very hazardous chemical.
EO + H2O = Ethylene Glycol
Ethylene glycol is the same as the antifreeze used in cars. Ethylene oxide sterilization can have the following effects on humans.
If EO contacts the patient’s blood, an anaphylactic reaction can occur.
There has been at least one recorded cardiac arrest related to EO exposure.
Reported injuries to employees include:
o Chemical burns
o Cataract formation
o Neurologic damage
o Leukemia
o Chromosomal aberrations
o Spontaneous abortions
Use of EO requires worker training, strict workplace safety guidelines, and periodic evaluation of exposures.
Low-Temperature Hydrogen Peroxide Gas Plasma Sterilization
Low-temperature hydrogen peroxide gas plasma sterilization is another sterilization method.
Gas plasma sterilization has the following features:
Plasma is a fourth state of matter distinguishable from a solid, liquid, or gas.
Gas plasmas are highly ionized gases, compounds of ions, electrons, and neutral atomic particles that produce a visible glow. Neon lights are an example of common, man-made plasma.
Hydrogen peroxide is bactericidal, virucidal, sporicidal, and fungicidal, even at low concentration and temperature.
Gas Plasma Sterilization Process
The low-temperature hydrogen peroxide gas plasma sterilization process has the following steps
1. Aqueous solution of hydrogen peroxide vaporized
The sterilization process begins with injection of hydrogen peroxide and water into a vacuum chamber. An aqueous solution of hydrogen peroxide is vaporized in the chamber surrounding the items to be sterilized. The hydrogen peroxide is transferred into the vaporizer, where water is removed from the solution. The sterilization chamber pressure is reduced, and the concentrated hydrogen peroxide is transferred to the chamber.
2. Electrons and other particles are accelerated.
A radiofrequency-induced electrical field accelerates electrons and other particles.
3. Collisions initiate reactions
The accelerated particles collide with each other, creating a plasma cloud.
4. Interaction with cell membranes
The plasma cloud interferes with the cell membranes, enzymes, and nucleic acids of microorganisms.
5. Disruption of life functions
Interaction of the plasma cloud with the cell membranes disrupts the life functions of the microorganisms.
6. Sterile product
A sterile product is rendered.
The efficacy of the cycle is monitored by several methods:
The sterilizer runs on a fixed, automatic cycle controlled by a microprocessor.
At the end of each cycle, a record of the process parameters is printed.
If any process parameter does not meet its acceptable limit, the cycle is canceled.
Biological and chemical indicators are also used to monitor sterilization.
Advantages of the Low-Temperature Gas Plasma Sterilization Process
There are many advantages to the low-temperature gas plasma system of sterilization over other methods of sterilization:
The total processing time is reduced, thus reducing turnover time. Check manufacturer's written directions for required cycle times
The end product is oxygen and vaporized moisture preserving the environment; therefore, no exhaust monitoring is required.
It does not leave a toxic residue.
The plasma is noncorrosive, allowing rigid endoscopes, fiberoptic cables, defibrillator paddles, metal, and hinged instruments to be processed.
The plasma system uses low temperatures.
Items are wrapped in specific materials; therefore, they can be stored for future use.
Because of its size, the unit can be installed and relocated easily.
Limitations of the Gas Plasma Sterilization Process
The low-temperature gas plasma sterilization process also has some limitations:
The rapid cycle of the plasma sterilization systems precludes the use of cellulose (paper), linens, and liquids.
Lumen sizes also will dictate whether an item can be processed by this system.
Refer to the manufacturer's written directions for channel diameter and length requirements.
It requires the use of a biological indicator made specifically for the low-temperature plasma sterilization unit.
Pathogen Description
Recording charts and graphs Recording charts and graphs are a permanent record of both the sterilizing time and the temperature attained in the exhaust line of the sterilizer. The charts and graphs must be dated, identified by sterilizer number, and carefully examined after each sterilizer run and again before refilling.
Chemical indicators Chemical indicators are physical or chemical devices employed to monitor one or more sterilization parameters for detecting failures in packaging, loading, sterilizer performance, or steam characteristics. The indicator gives instant, visual assurance that proper conditions for sterilization were present and an item has been subjected to one or more parameters of the sterilization process. They do not prove sterility of an item. The chemical indicator is placed in the center of packages, among instruments in a tray, or other locations where it is necessary to ensure steam penetration and correct temperature attainment.
Temperature-specific indicators Temperature-specific indicators are designed to reveal the attainment of a specific minimum temperature at its location within the sterilizer chamber or load. These indicators do not require the presence of moisture. Temperature-specific indicators can consist of a chemically treated paper indicator, which changes colors when exposed. Autoclave tape is one example and is useful in identifying packages and articles that have been processed.
Integrator The integrator is a multi-parameter indicator that has been designed to provide an integrated response to defined combinations of temperature, time, and presence of steam. They are sterilant-specific. Integrators are based on a chemical and/or physical change that also results in a color change or migration of a chemical along a wick. This is a more sophisticated and accurate test of the sterilization process.
Daily function test A daily function test or a Bowie-Dick test must be performed daily on high-vacuum/pre-vacuum sterilizers. The purpose of this test is to determine the adequacy of the mechanical air removal during the pre-vacuum stage. The test pack should always be placed into an empty sterilizer over the drain (not in a pan). The test results must read in accordance with the manufacturer’s recommendations. Failure of this test requires checking the sterilizer, identifying the cause of the malfunction and correcting it before the sterilizer can be returned to service.
Biological Monitoring
Biological monitoring is used to document the efficacy of specific sterilization cycles, not the sterility of the item. Biological indicators (BI) contain known populations of microorganisms, in or on a carrier, that have specific resistance to the mode of sterilization being monitored.
Sterilizer efficacy should be monitored at regular intervals with the appropriate BI. Each load containing implantable devices should be monitored, and when possible, the implantable device should be quarantined until the results of the biological indicator are available.
The following microorganisms are commonly used in biological indicators.
Geo-bacillus stearothermophilus is used for:
Steam sterilizers
Hydrogen peroxide gas plasma sterilizers
Ozone sterilizers
Liquid peracetic acid sterilizers
Bacillus atropheus is used for:
Ethylene oxide sterilizers
Dry-heat sterilizers
Responses
After exposure to the sterilization cycle and incubation, the absence of growth indicates that the appropriate parameters have been met. No growth, no color change, or no fluorescence demonstrates a negative response.
A positive response is demonstrated by the appearance of an either cloudy (turbid) growth medium, colony-forming units, a color change, or fluorescence.
Negative response
No growth
No color change
No fluorescence Positive response
Cloudy (turbid) growth
Colony-forming units
Color change
Fluorescence
A positive BI indicates a possible sterilization process failure. The following steps should be followed when a positive response is encountered:
Positive biological indicator test results should be reported immediately.
All products processed since the last negative BI may be recalled.
An investigation must take place to determine what caused the failure before the sterilizer can be used and the load either released for use or reprocessed.
Retesting of the sterilizer is required if any sterilizer repairs occur or if the steam supply has been interrupted.
Sterilization records for all sterilizers are maintained for each cycle for the time indicated by the state’s Statute of Limitations. Record keeping includes results of daily function tests, results on biological monitoring, autoclave charts and graphs, and records indicating load contents and load control numbers.
Types of Surgical Implants
Many different types of implants are used in surgery. Almost every surgical specialty uses some type of implant. Some of the different types of surgical specialties and implants include the following:
Type of surgery Surgical specialties and implants
Cardiac/Vascular Surgery Valve prostheses, artificial and human hearts, arterial tube grafts, Teflon and Dacron patches, vena cava filters, pacemakers, and implantable cardioverter defibrillators
Ears, Nose, and Throat (ENT) Cochlear implants, myringotomy tubes, stapedectomy prostheses, and ossicular prostheses
General Surgery Synthetic mesh
Neurosurgery Aneurysm clips and ventriculoatrial shunts
Ophthalmic Intraocular lens implants (IOL), scleral buckles/bands, human donor corneas, and artificial eyes
Orthopedic Ankle, finger, hip, knee, shoulder, and toe joint implants; metal plates, screws, nails, pins, and bone allograft
Plastic/Reconstructive Arch bars, breast implants, chin implants, stainless steel wire, and mini-plating devices
Genitourinary Artificial urinary sphincters and penile prostheses
Requirements of Perioperative Documentation
According to AORN Perioperative Standards and Recommended Practices for implant documentation, perioperative documentation should include the following:
Placement and location of implants
Name of the manufacturer
Lot and serial numbers
Type and size of implant
Expiration dates as appropriate
Any other information required by the US Food and Drug Administration
Sterilization Documentation
Sterilization records should include information on each load, including:
the patient receiving the item(s);
the item(s) processed;
the cycle parameters used (eg, temperature, duration of cycle);
the date and time the cycle is run;
the operator information; and
the reason for immediate-use steam sterilization.
Tracking System Guidelines
The Medical Device Tracking Requirements were published in the Safe Medical Devices Act of 1990. An update was printed on April 1, 1998. These regulations require tracking of designated medical devices from the manufacturer to the person for whom the device is indicated. Facilities are required to develop a tracking system to comply with these regulations.
Upon acquisition, the facility is required to track the devices from the manufacturer to the patient and back to the manufacturer upon implantation. Records must be kept and made available upon audit to the manufacturer or FDA. All records must be maintained for the useful life of each tracked device.
Drug Administration (FDA) defines an implant as a permanently implantable device. 21 CFR 821.3(f) states, “Permanently implantable device means a device that is intended to be placed into a surgically or naturally formed cavity of the human body to continuously assist, restore, or replace the function of an organ system or structure of the human body throughout the useful life of the device. The term does not include any device which is intended and used for temporary purposes or which is intended for explantation.” The FDA time definition is materials that will remain in the body for greater than one year. Implants that are not intended to be in the body for more than one year should be documented on the perioperative record, but they are not required to be tracked by FDA.
Surgical Tissue Banking
Surgical tissue banking encompasses the retrieval, processing, preserving, and storing of selected human tissue. Some implants used in surgical procedures are human tissue and/or bone and require refrigeration or freezing. Some facilities have established on-site tissue banks in order to maintain these types of implants on the premises.
FDA and AATB Regulations
The FDA (Food and Drug Administration) and the AATB (American Association of Tissue Banks) have established regulations for providing safe, reliable, and biologically useful grafts.
These regulations establish performance requirements for donor selection as well as for other activities regarding transplantable human musculoskeletal, skin, reproductive and cardiovascular cells and/or tissue. Licensed tissue banks must comply with these regulations. These activities include:
Processing
Storage
Packaging
Labeling
Distribution
Transportation
Storage Requirements
One important requirement for surgical tissue banking addresses the storage environment for the tissue. The AATB is concerned with issues such as access, daily temperature monitoring and recording, calibration of the refrigerator/freezer, and alarm systems. Sometimes these requirements are difficult for facilities to accomplish.
AORN’s Perioperative Standards and Recommended Practices address these requirements and can be used as a guide for personnel who are involved in the procuring, processing, preserving, and storing of human tissue.
Remember, these tissues are considered implants and should be documented in the perioperative record in the same manner as we have already covered.
Storage of Sterile Items
Shelf life is defined as the period of time that sterility is generally maintained and is related to the capability of the packaging material and the storage conditions to protect the sterility of the packaged item after sterilization. Shelf life should be considered event-related, not time-related.
The length of time an item is considered sterile depends on factors that include the following:
Type and configuration of packaging materials used
Number of times a package is handled before use
Number of personnel who may have handled the package
Storage on open or closed shelves
Condition of the storage area, that is, cleanliness, temperature, humidity, air exchange
Use of sterility maintenance covers (dust covers) and method of sealing
Conditions during transport
Correct quantity and quality of supplies should be readily available to meet demands. Stock rotation should follow the principles of FIFO: first in/first out.
Sterile packages should be stored under environmentally controlled conditions. Controlled conditions reduce the risk of contamination.
The temperature in the sterile storage areas should not exceed 24° C (75° F).
The storage area should have at least four air exchanges per hour.
Relative humidity should be controlled, not to exceed 70%.
Traffic should be controlled to limit access to those trained in handling sterile supplies.
Supplies should be stored in a manner that allows adequate air circulation, ease of cleaning, and compliance with local fire codes.
Sterile items should be stored at least eight to 10 inches above the floor, at least18 inches below sprinkler heads, and at least two inches from outside walls.
Outside shipping containers should not be allowed in the sterile storage area because they serve as generators of, and reservoirs for, dust.
Contamination of Sterile Items
A sterile item can become contaminated in a number of ways:
Airborne bacteria and dust can be forced into a package by incorrect or excessive handling, poor storage facilities, or improper techniques.
An aerosol or bellows effect occurs by the squeezing action of the hands each time the package is handled or by dropping a package onto the floor or hard surface.
Bacteria can enter packages through ruptured seals, small breaks, and tears not easily detected.
Moisture can be absorbed into pervious packages if a package is placed on a wet or damp surface, by carrying a package tightly under the arm where it can contact a warm, wet armpit, and handling the package with wet or sweaty hands.
Disinfection
There are three levels of disinfection.
High-level disinfection
Kills all vegetative forms of bacteria, all fungi, and all viruses
Highly resistant bacterial spores might survive
Does not inactivate the virus-like prion thought to cause CJD or vCJD
Intermediate-level disinfection
Inactivates Mycobacterium tuberculosis, vegetative bacteria, most viruses, and most fungi
Does not kill bacterial spores Disinfection is defined as any process, chemical or physical, that kills or destroys pathogenic microorganisms on inanimate surfaces and objects.
Low-level disinfection
Kills most bacteria, some viruses, and some fungi
Cannot be relied upon to kill resistant microorganisms such as tubercle bacilli or bacterial spores
Spaulding Classification System
Unlike sterilization, disinfection rarely kills spores. It reduces the risk of microbial contamination, but does not provide the same level of assurance as sterilization. Items to be disinfected should be categorized as critical, semicritical, or noncritical using the Spaulding Classification System.
Critical items
Critical items include instruments or objects that are introduced into the human body, either into or in contact with the blood stream or normally sterile areas of the body, for example, surgical laparoscopic instruments, implants, cardiac catheters.
Disinfection level
Items that fall into this category must be sterilized before use.
If all microorganisms are not removed, including bacterial spores, the risk of infection is high.
If sterilization is not feasible, these items must receive high-level disinfection.
Semicritical items
Semicritical items are those that come in contact with mucous membranes and do not ordinarily penetrate body surfaces; for example, endoscopes that are passed through natural body orifices, anesthesia equipment (including laryngoscope handles and blades), and respiratory equipment.
Disinfection level
Items falling into this category should receive high-level disinfection. The rationale is that an intact mucous membrane usually resists common bacterial spores but may be susceptible to other organisms.
Noncritical items
Noncritical items are those that come in contact with the patient’s unbroken skin, for example, blood pressure cuffs, bedpans, pulse oximeters.
Disinfection level
These items should receive intermediate or low-level disinfection because the intact skin serves as a barrier to most microorganisms.
Chemical High-level Disinfection
Chemical high-level disinfection is achieved by immersing an item for a specified period in a chemical agent that has been cleared by the US Food and Drug Administration (FDA) as a disinfectant/sterilant.
A few guidelines need to be followed during chemical disinfection.
When selecting a chemical agent to be used for disinfection, materials compatibility and the factors influencing efficacy should be considered.
Items should be thoroughly cleaned and decontaminated before disinfection. Debris, blood, mucous, and tissue will interfere with the action of the disinfectant.
After the cleaning process, the instruments should be rinsed and dried before placing in the chemical disinfectant.
o Rinsing removes any residual detergent
o Drying prevents dilution of the disinfectant when the instruments are placed in the solution
Items to be chemically disinfected should be completely immersed in the disinfectant solution according to the recommendations of established infection control practice and/or product manufacturer.
The chemicals commonly used for high-level disinfection are:
2% glutaraldehyde
Ortho-Phthalaldehyde (OPA)
2% Glutaraldehyde
A 2% alkaline glutaraldehyde formula is a chemical sterilant that is used for high-level disinfection, but it is also capable of sterilizing (10 hours). To be effective during use, alkaline glutaraldehyde solutions are activated by adding the contents of the activator vial to the container of glutaraldehyde solution. The solution changes color, indicating that it has been activated and is ready for use. Most chemical germicides are effective for only a specific period of time and should be labeled with an expiration date. At regular intervals, as prescribed by the manufacturer and whenever excessive dilution or heavy contamination is suspected, the glutaraldehyde must be tested for its strength and discarded if necessary.
Although effective against microorganisms in less than 10 minutes, glutaraldehyde requires somewhat longer time intervals to inactivate certain species of mycobacteria. The Association for Professionals in Infection Control (APIC) has published guidelines stating that the exposure needed to achieve high-level disinfection in 2% glutaraldehyde is a minimum of 20 minutes at 68° F. (20° C.) when used on meticulously cleaned items. After the recommended soaking time has elapsed, instruments are removed from the solution, using sterile technique. Special care should be taken to rinse the items with sterile water, ensuring that lumens are flushed, and the items are dried. Rinsing removes the toxic and irritating residue and prevents tissue damage.
Two percent (2%) glutaraldehyde should be kept in a covered plastic container and used in a well-ventilated area in order to prevent exposure to the noxious fumes. When handling chemical disinfectants personnel should wear protective apparel such as eyewear, masks, moisture-repellent or splash-proof skin protection, and gloves designed for handling chemicals. 100% nitrile rubber of 100% butyl rubber gloves must be used when handling glutaraldehyde.
Ortho-Phthalaldehyde (OPA)
Ortho-Phthalaldehyde (OPA) is a high-level disinfectant used for heat-sensitive items. OPA requires no activation to make it ready for use. OPA has demonstrated efficacy in the presence of organic soil contamination and microbiological burden during reuse.
Items and their lumens must be thoroughly cleaned, rinsed, and dried. Items must be immersed, filling all lumens, and eliminating air pockets for a minimum of 12 minutes at 68° F. or higher to destroy all pathogenic microorganisms, including vegetative organisms such as Mycobacterium bovis and Pseudomonas aeruginosa, pathogenic fungi, and viruses.
OPA should be used in a well ventilated area and in closed containers with tight-fitting lids. Items soaked in OPA require three separate, large-volumes, sterile-water immersion rinses. Personnel should wear protective apparel such as eyewear, masks, moisture-repellent or splash-proof skin protection, and gloves designed for handling chemicals. OPA may temporarily stain exposed skin or clothing. Direct contact with eyes may cause irritation.
Once poured from the bottle, OPA may be used for a period up to 14 days. The manufacturer recommends that the solution be tested before each use to ensure that the appropriate concentration of OPA is present. Once opened, the unused portion of the solution may be stored in its original container for up to 75 days.
Recommended Practice Statement
Cleaning and Caring for Surgical Instruments and Powered Equipment –
Recommended Practice XIII "Powered surgical instruments and all attachments should be decontaminated, lubricated, assembled, sterilized, and tested before use according to the manufacturers’ written instructions
Cleaning and Caring for Surgical Instruments and Powered Equipment -
Recommended Practice IV
"Instruments should be kept free of gross soil during surgical procedures."
Cleaning and Caring for Surgical Instruments and Powered Equipment –
Recommended Practice V "Cleaning and decontamination should occur as soon as possible after instruments and equipment are used."
Cleaning and Caring for Surgical Instruments and Powered Equipment -
Recommended Practice XI "Surgical instruments should be inspected for cleanliness and proper working order after decontamination."
Cleaning and Caring for Surgical Instruments and Powered Equipment -
Recommended Practice XVII
"Special precautions should be taken to minimize the risk of transmission of prion diseases."
[Show More]