Clinical Chemistry > EXAMs > AAMC MCAT Exam 4- Chem/Phys 2022/2023 with complete solution (All)
AAMC MCAT Exam 4- Chem/Phys 2022/2023 with complete solution
Document Content and Description Below
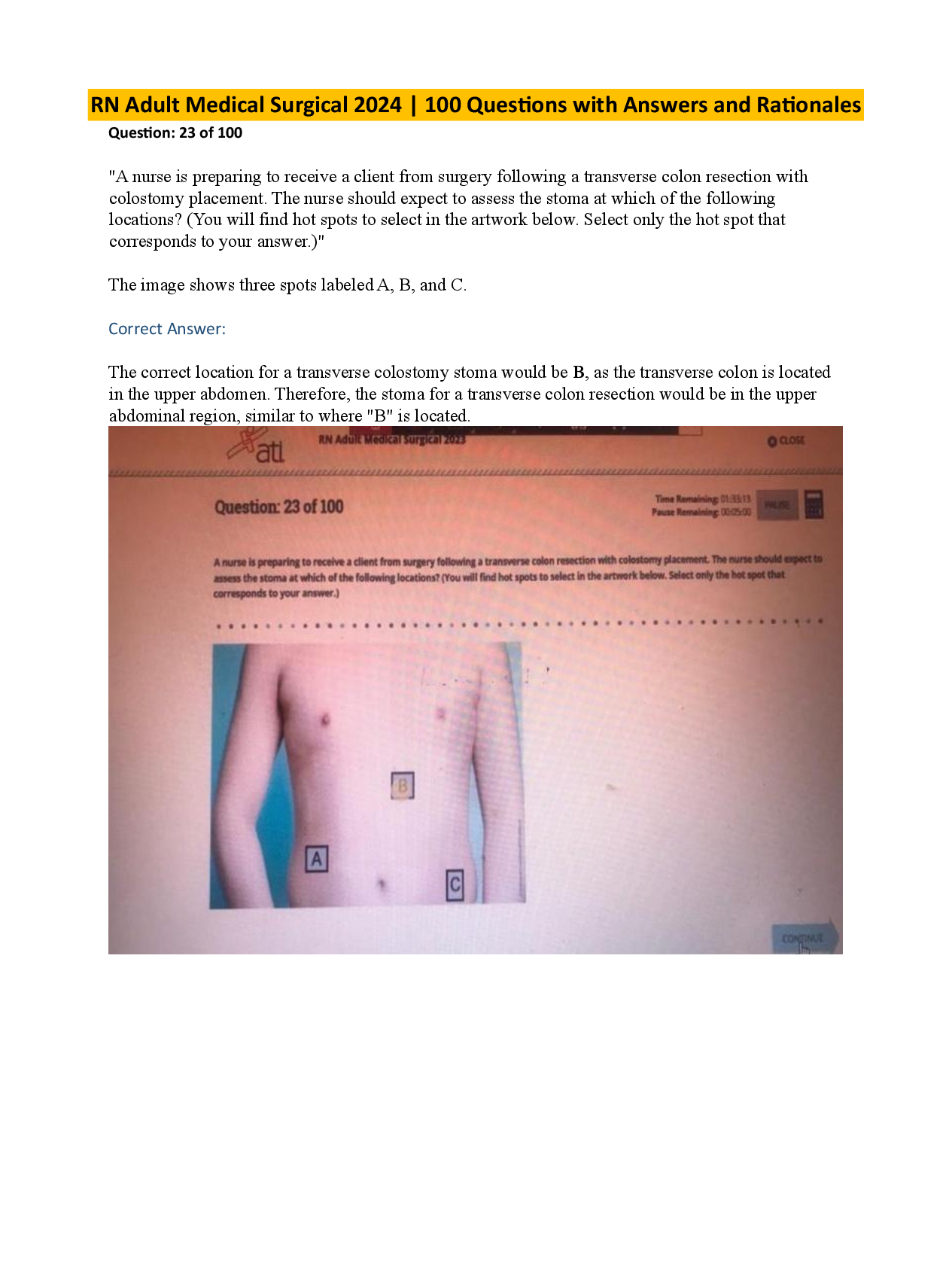
Limestone does NOT decompose when heated to 900 K because, at 900 K, ΔH is: A. positive and less than TΔS. B. positive and greater than TΔS. C. negative and less than TΔS. D. negative and ... greater than TΔS. - ANSWER B. positive and greater than TΔS. The reaction does not occur (is not spontaneous). This indicates that ΔG = ΔH - TΔS > 0. From inspection of the reaction, it can be concluded that ΔS > 0 (a gas evolves). Consequently, ΔH > TΔS explains why the reaction does not occur. According to Table 1, what is the concentration of the glucose in the blood from which the diluted sample was taken? A. 60 mg/dL B. 90 mg/dL C. 120 mg/dL D. 150 mg/dL - ANSWER D. 150 mg/dL From Table 1, the glucose concentration in the diluted sample is (0.20/0.24) × 6.0 mg/dL = 5.0 mg/dL. The blood then has a glucose concentration of 30 × 5.0 mg/dL = 150 mg/dL. Which of the following oxidative transformations is unlikely to occur? A. A primary alcohol to an aldehyde B. A tertiary alcohol to a ketone C. An aldehyde to a carboxylic acid D. A secondary alcohol to a ketone - ANSWER B. A tertiary alcohol to a ketone Oxidation of tertiary alcohols is difficult because it involves C-C bond breaking. What is the effect produced by the PRK technique designed to correct nearsightedness? A. The density of the cornea is increased. B. The radius of curvature of the cornea is increased. C. The index of refraction of the cornea is increased. D. The thickness of the cornea at the apex is increased. - ANSWER B. The radius of curvature of the cornea is increased. in passage, it says nearsightedness flattens the cornea. Flattening the cornea would increase the radius of curvature. The use of pulsed laser radiation in the PRK procedure, as opposed to continuous laser radiation, allows the cornea to: A. absorb more radiation. B. change its index of refraction. C. increase the area exposed to radiation. D. maintain a lower average temperature. - ANSWER D. maintain a lower average temperature. The pulsed laser radiation interacts with the cornea for a shorter time than a continuous laser radiation, thus less heat is transferred to the cornea. This allows the cornea to maintain a lower average temperature by cooling off between pu Which conclusion can be drawn from the experimental results described in the passage? A. Compound 1 selectively sensitizes cancer cells to chemotherapeutic action by Compound 2. B. Compound 1 sensitizes all cells to chemotherapeutic action. C. Compound 2 induces apoptosis in cancer cells only in conjunction with treatment with Compound 1. D. Compound 2 inhibits the NF-κB signaling pathway, which leads to apoptosis. - ANSWER A. Compound 1 selectively sensitizes cancer cells to chemotherapeutic action by Compound 2. The results shown in Figure 1 and Table 1 show that treatment of cancerous cell lines with Compound 1 sensitizes cancer cells to chemotherapeutic action as a result of exposure to Compound 2. What is the concentration of the acute leukemia CC95 Combination value in micromolar (μM) units? A. 6 × 10-10 μM B. 6 × 10-7 μM C. 6 × 10- 4 μM D. 6 × 10-2 μM - ANSWER The CC95 Combination value for acute leukemia cells was 0.6 nM, or 0.6 × 10-9 M. This corresponds to 0.6 × 10-9 M × (1 μM/1 × 10-6 M) = 0.6 × 10-3 μM = 6 × 10-4 μM. Which transition produces a photon with the longest wavelength? A. Transition 1 B. Transition 2 C. Transition 3 D. Transition 4 - ANSWER B. Transition 2 The longest wavelength has the smallest photon energy (closest energy levels) Which of the following functional groups is present in pyrrolizidine (Figure 1)? A. An amide B. An amine C. An imine D. A carbamate - ANSWER B. An amine (R3N) where R=H Compound 3 is prepared from Compound 2 (Figure 2) by: A. reduction of the ketone and lactonization of the gamma-hydroxyester. B. hydrolysis of one ester and formation of an acetal from the ketoacid. C. reduction of one ester and formation of an acetal from the gamma-hydroxyketone. D. reduction of one ester and the ketone followed by dehydration to a ketoether. - ANSWER A. reduction of the ketone and lactonization of the gamma-hydroxyester. What is the approximate concentration of reaction product in a solution that has an absorbance of 0.7 at pH 6.0? A. 0.50 μM B. 500 μM C. 5 mM D. 500 mM - ANSWER B. 500 μM Based on the equation A = εbc given in the passage, the concentration c is the absorbance A divided by the absorptivity ε in a 1 cm path length cell. 0.7 divided by approximately 1400 (from table) gives 500 μM. Given that Tris has a pKa of 8.07, for how many of the experiments would Tris have been an acceptable buffer? [Show More]
Last updated: 3 years ago
Preview 1 out of 9 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Also available in bundle (1)
Click Below to Access Bundle(s)

AAMC MCAT Exam 4-BIO-chem-Psych 2022/2023- full pack solution
AAMC MCAT Exam 4- Bio 2022/2023 with complete solution AAMC MCAT Exam 4- Chem/Phys 2022/2023 with complete solution AAMC MCAT Exam 4- Psych 2022/2023 with complete solution
By MARKALLAN 3 years ago
$13.5
3
Reviews( 0 )
$8.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Aug 24, 2022
Number of pages
9
Written in
All
Additional information
This document has been written for:
Uploaded
Aug 24, 2022
Downloads
0
Views
190