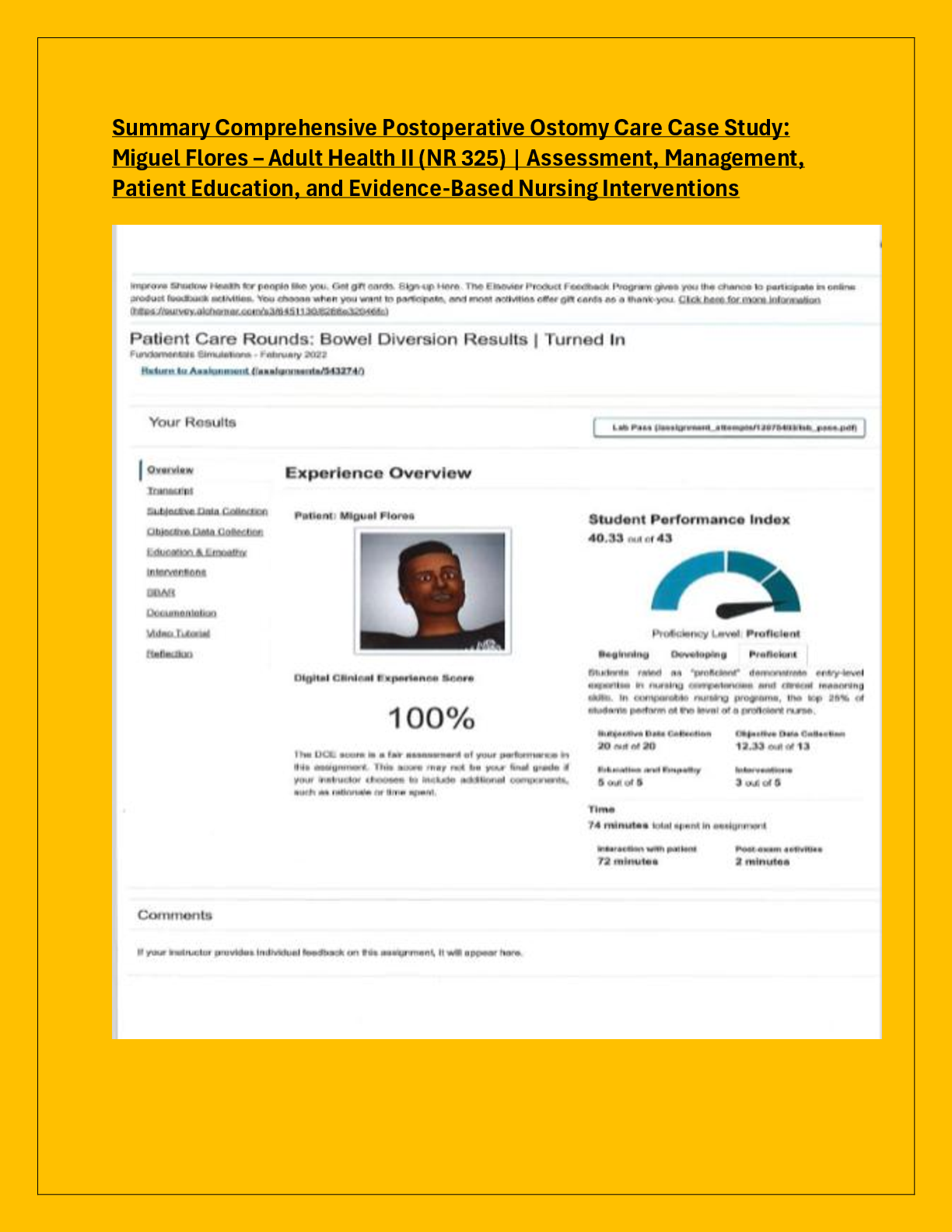
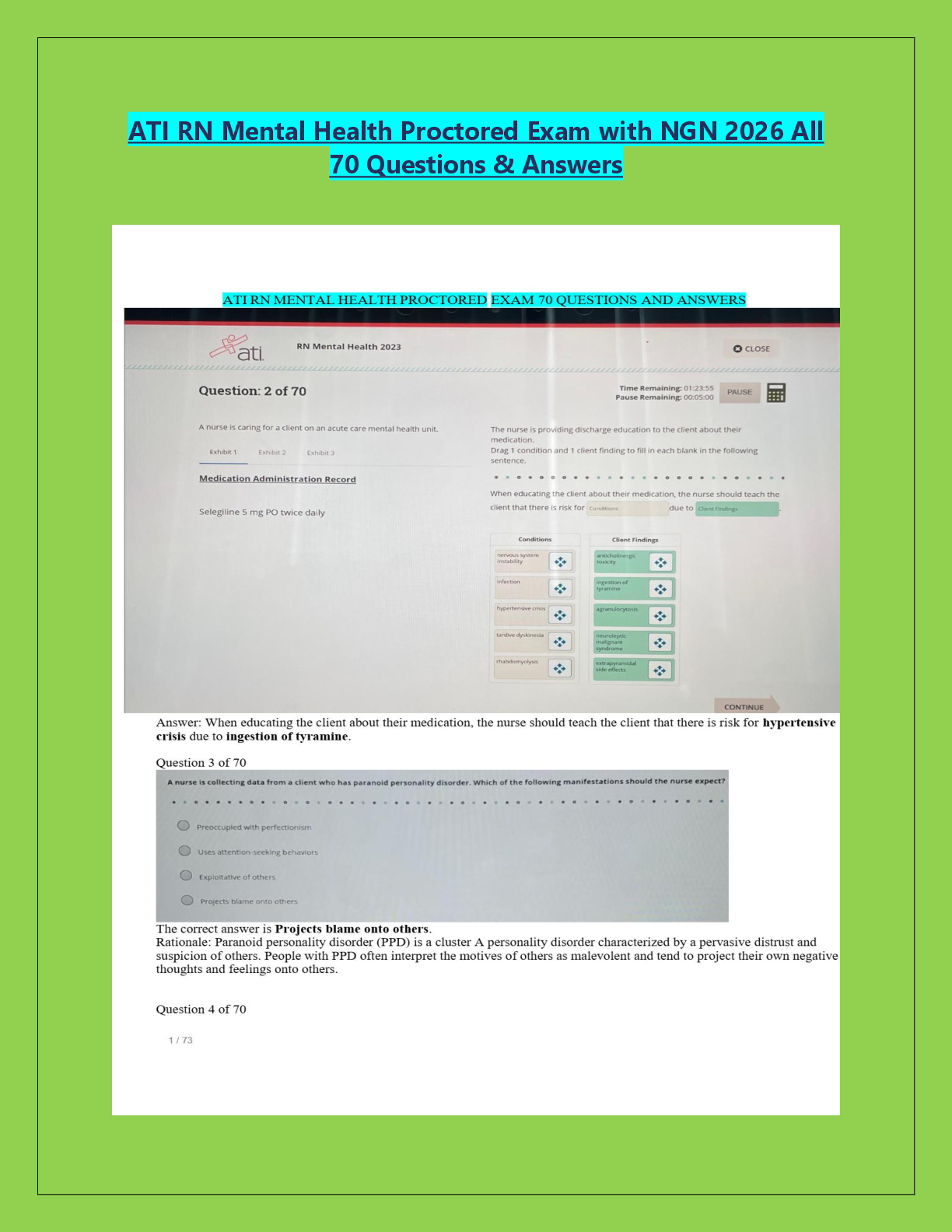
BioChemistry > EXAMs > Biochemistry Final Exam Sample Questions and answers | 16 pages (All)
Biochemistry Final Exam Sample Questions and answers | 16 pages
Document Content and Description Below
Which of the following is true about the properties of aqueous solutions? A) Hydrogen bonds form readily in aqueous solutions. B) Charged molecules are generally insoluble in water. C) An increase ... in pH from 5.0 to 6.0 reflects an increase in the hydroxide ion concentration ([OH–]) of 20%. D) A decrease in pH from 8.0 to 6.0 reflects a decrease in the proton concentration ([H+]) by a factor of 100. 2. A hydronium ion: A) is a hydrated proton. B) is a hydrated hydrogen ion C) has the structure H3O+. D) is the usual form of one of the dissociation products of water in solution. E) All of the above are true. 3. One hundred mL of 0.1 M NaOH is added to 55 mL of 0.2 M lactic acid. (The pKa of lactic acid is 4.1.) The resulting mixture has a pH close to: A) 2. B) 3. C) 4. D) 5. E) 6. 4. Name and briefly define four types of noncovalent interactions that occur between biological molecules. 5. For each of the pairs below, circle the conjugate base. RCOOH RCOO– RNH2 RNH3 + H2PO4 – H3PO4 H2CO3 HCO3 – Page 2 6. Give the general Henderson-Hasselbalch equation and sketch the plot it describes (pH against amount of NaOH added to a weak acid). On your curve label the pKa for the weak acid, and indicate the region in which the buffering capacity of the system is greatest. [Show More]
Last updated: 3 years ago
Preview 1 out of 16 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$11.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Aug 24, 2022
Number of pages
16
Written in
All
Additional information
This document has been written for:
Uploaded
Aug 24, 2022
Downloads
0
Views
182




.png)