Chemistry > QUESTIONS & ANSWERS > Boyles Law Questions | questions and answers (All)
Boyles Law Questions | questions and answers
Document Content and Description Below
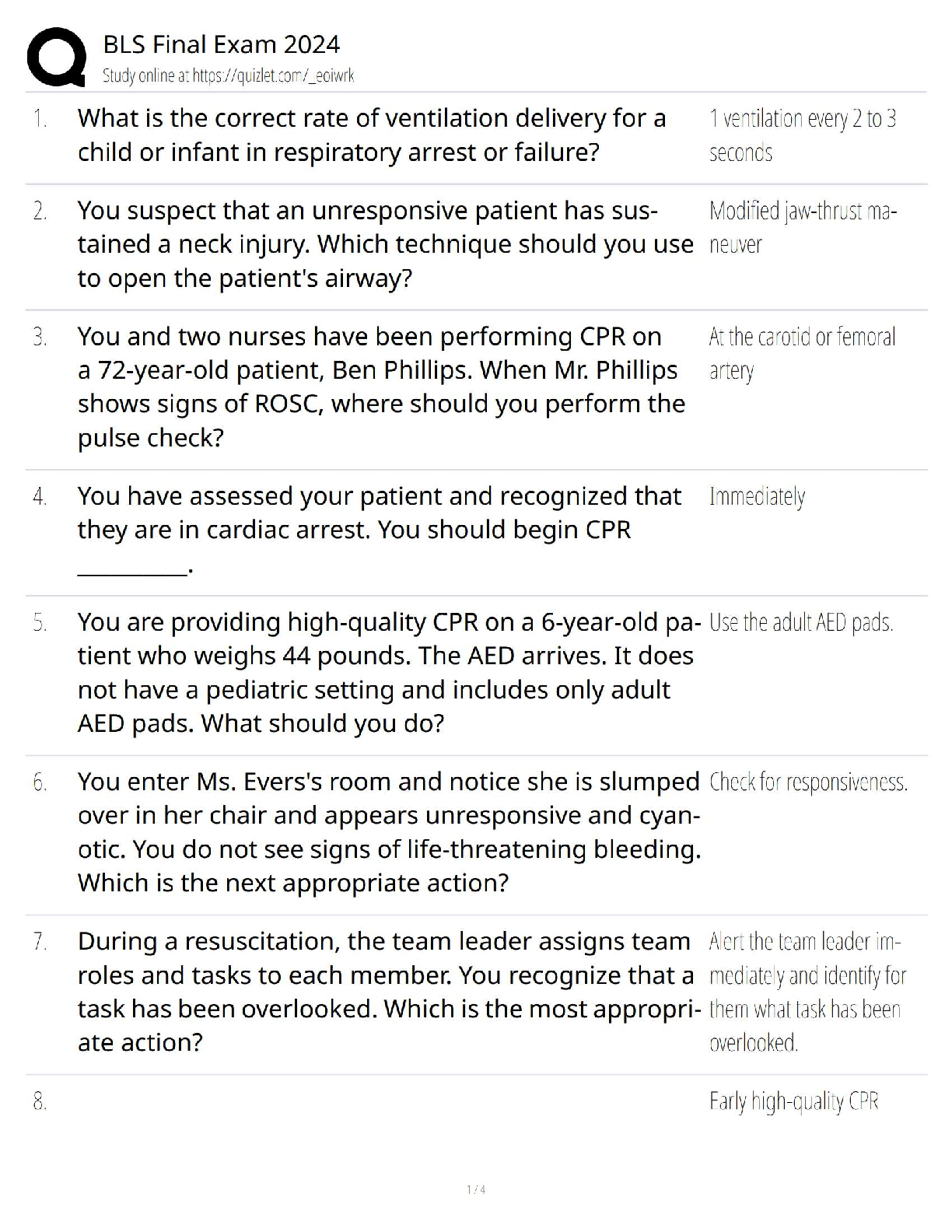
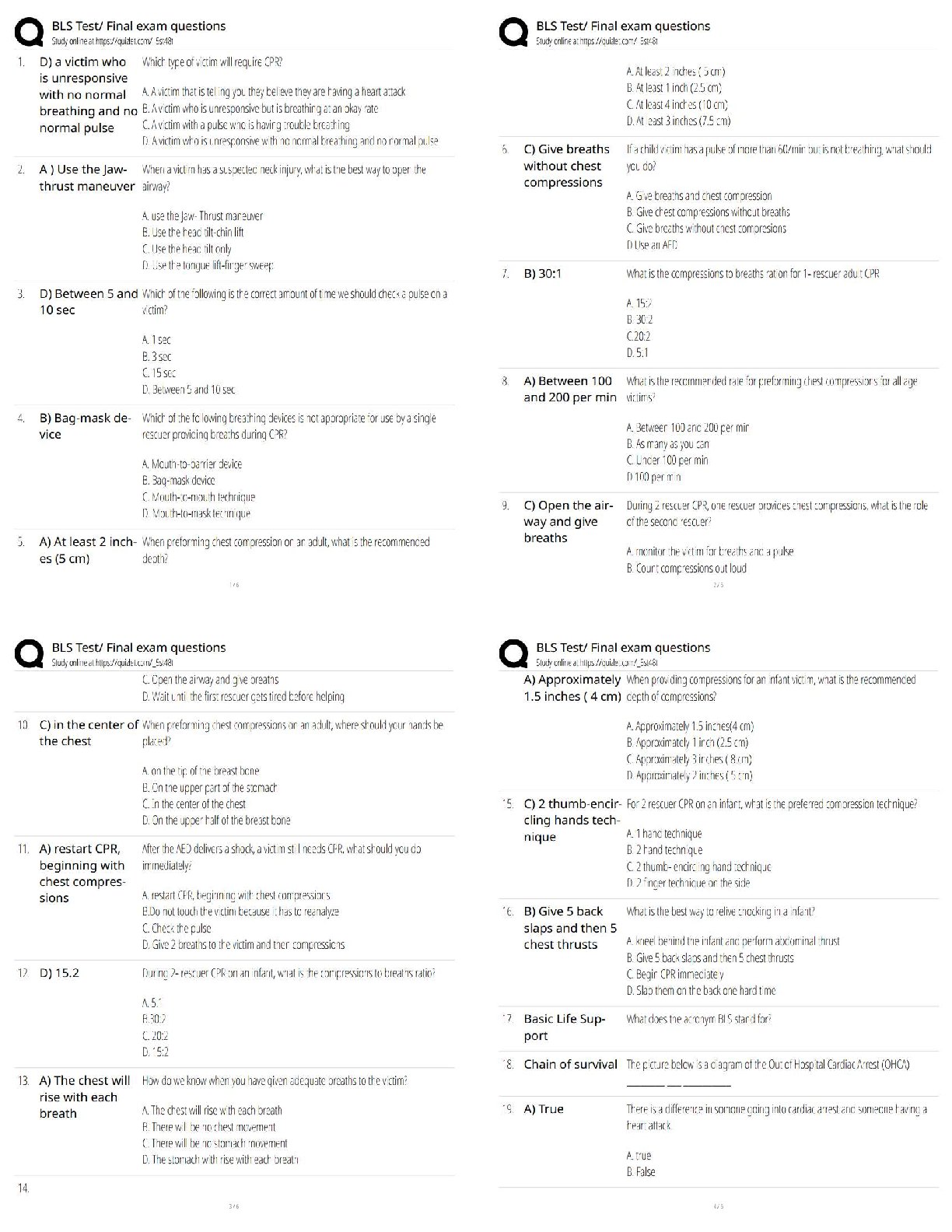
Does your experimental data for propane verify Boyle’s Law? Explain. Yes, it does because Boyle’s Law states that pressure and volume are inversely proportional meaning when pressure increase th ... e volume decreases, and when pressure decreases volume increases. That is was the experimental data for propane showed. 4. What pressure would the propane gas sample have at 75 mL? The pressure would be close to 1.89 atm. Experiment 2: Determine the Relationship Between the Volume and Pressure of Butane gas Lab Results 1. Record the pairs of data for pressure and butane volume in the table below. Volume of Gas (mL) Pressure (atm) 150 1.00 130 1.15 110 1.36 90 1.67 70 2.14 50 3.00 Data Analysis 2. Create and save a graph of pressure in atm (y-axis) versus the 1/volume of butane in 1/mL (xaxis). Click the graphing icon below to create your graph and enter the values for the 1/volume of butane as decimals, not fractions. [Show More]
Last updated: 3 years ago
Preview 1 out of 8 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$9.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Aug 26, 2022
Number of pages
8
Written in
All
Additional information
This document has been written for:
Uploaded
Aug 26, 2022
Downloads
0
Views
221


.png)
.png)
.png)
.png)
.png)
.png)

.png)