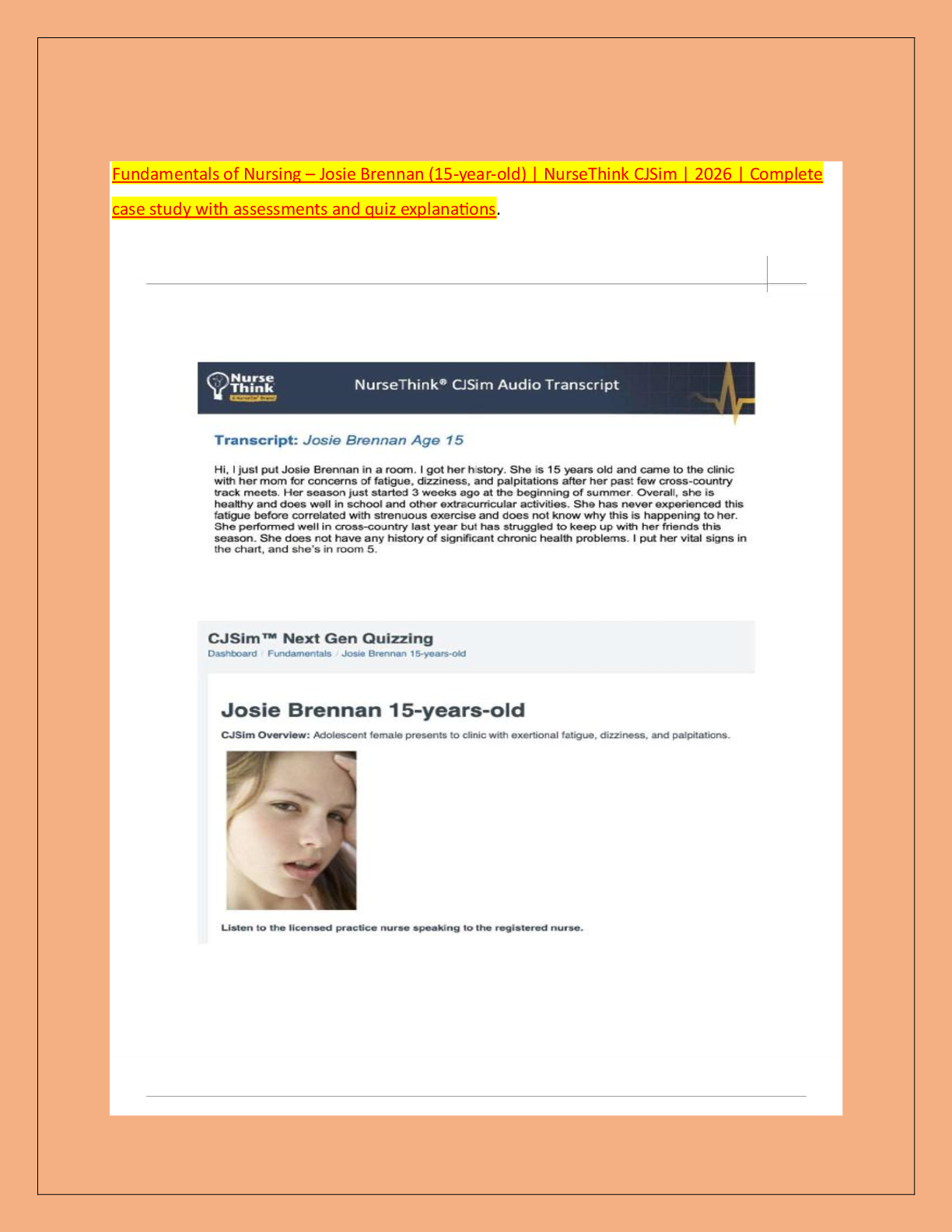
SCIENCE 101 > EXAM > ATI Teas Test C Latest Updated 2022 100% Pass (All)
ATI Teas Test C Latest Updated 2022 100% Pass
Document Content and Description Below
Mg(OH)2 + HCl -> The equation above is unbalanced. Which of the following shows the molar products of the neutralization reaction after balancing? A. 2MgCl2 + H2O B. 2MgCl2 + 2H2O C. MgCl2 + H2O ... D. MgCl2 + 2H2O ✔✔D. MgCl2 + H2O H+ + HCO3- <-> H2O + CO2 Based on the equation above occurring in blood, which of the following would result if the reaction is moving in a forward direction? A. Blood pressure will decrease B. The blood will become more acidic C. The blood will become more basic D. Proton concentration will increase ✔✔C. The blood will become more basic Which of the following is the collagen-rich material that connects muscles to bones? A. Tendon B. Ligament C. Reticular T [Show More]
Last updated: 3 years ago
Preview 1 out of 13 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$10.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Sep 26, 2022
Number of pages
13
Written in
All
Additional information
This document has been written for:
Uploaded
Sep 26, 2022
Downloads
0
Views
47



.png)





.png)