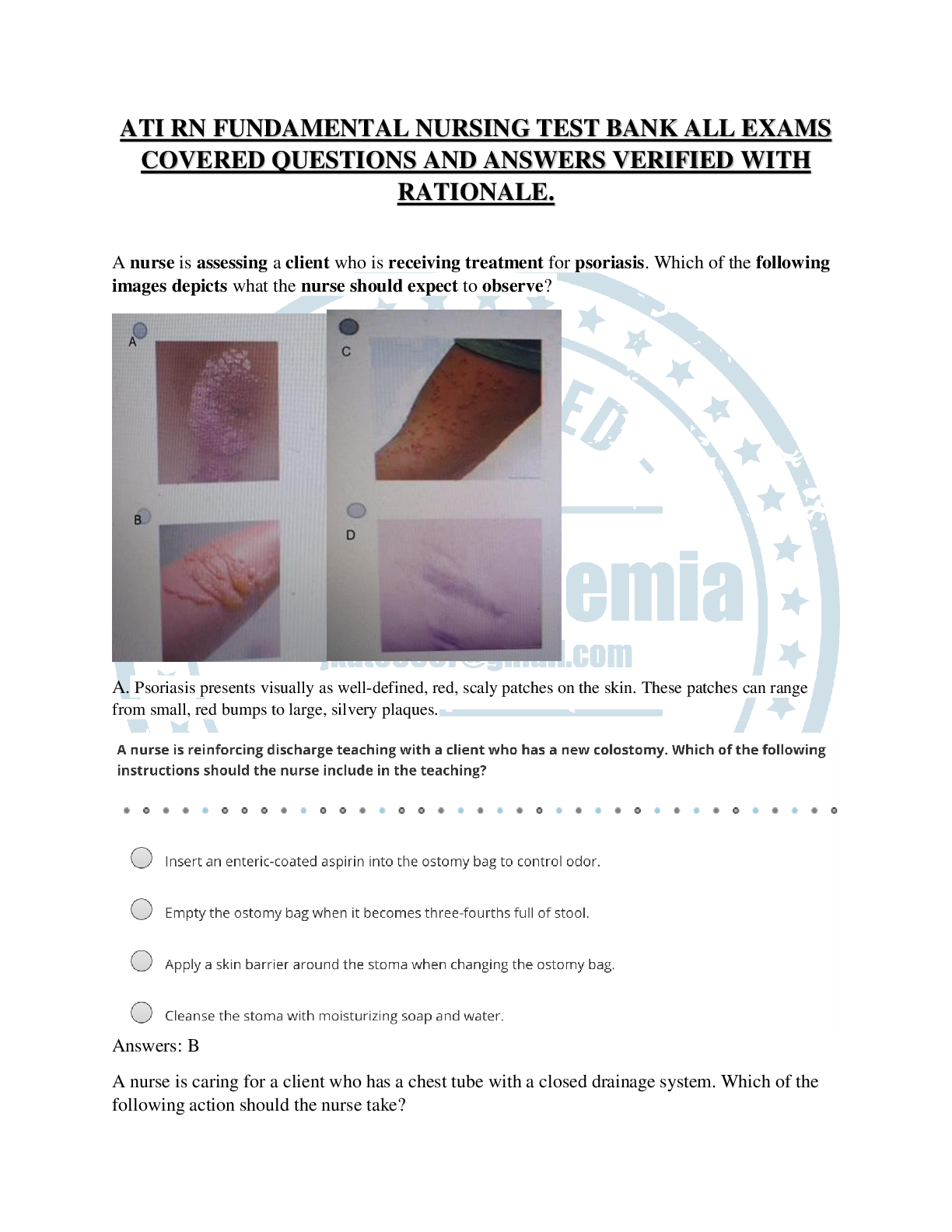
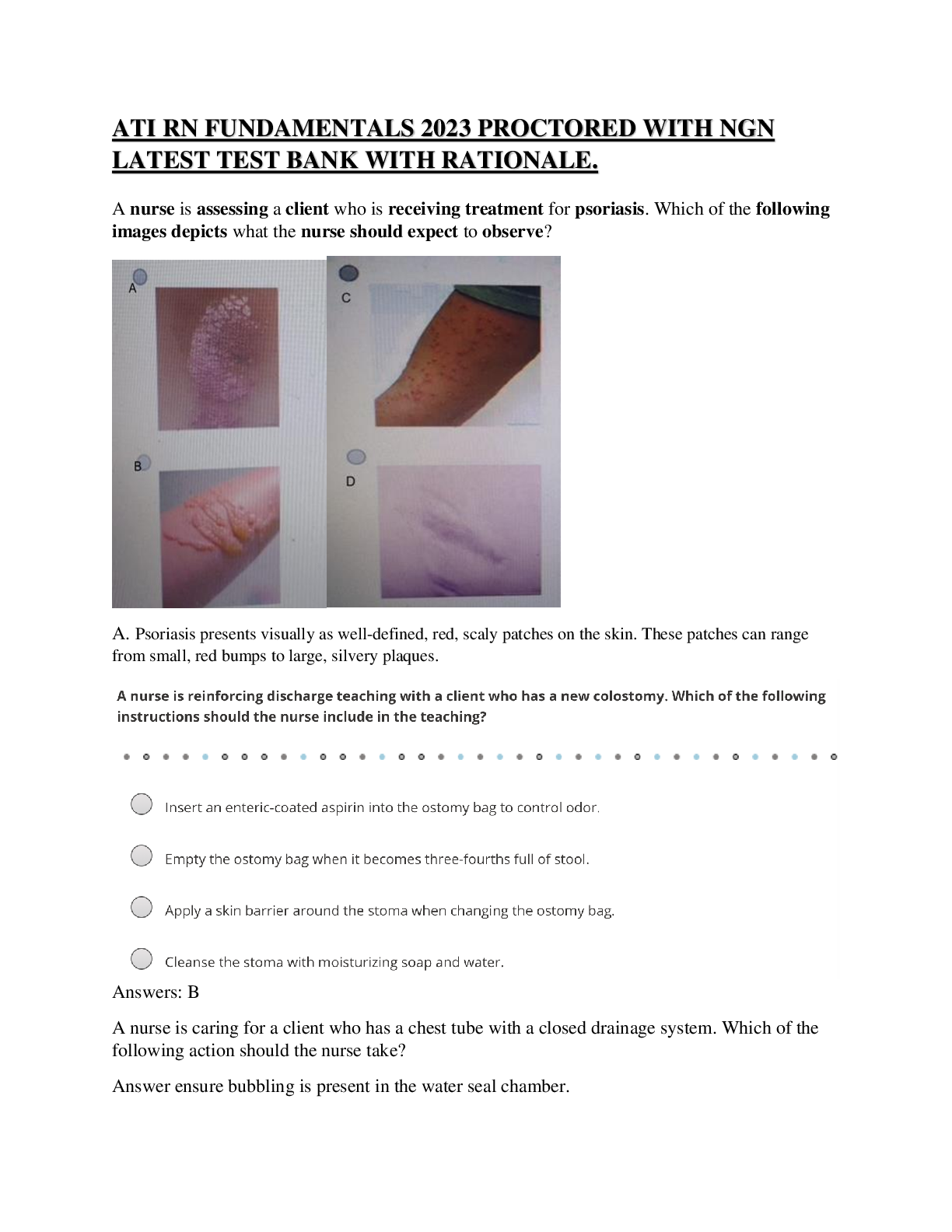
Portage Learning / CHEM 103 / Module 1 to 6 Exam
MODULE 1 EXAM
Question 1 Click this link to access the Periodic Table. This ma
...
Portage Learning / CHEM 103 / Module 1 to 6 Exam
MODULE 1 EXAM
Question 1 Click this link to access the Periodic Table. This may be helpful throughout the exam.
1. Convert 845.3 to exponential form and explain your answer.
2. Convert 3.21 x 10-5 to ordinary form and explain your answer.
1. Convert 845.3 = larger than 1 = positive exponent, move decimal 2 places
= 8.453 x 102
2. Convert 3.21 x 10-5 = negative exponent = smaller than 1, move decimal 5 places = 0.0000321
Question 2 Click this link to access the Periodic Table. This may be helpful throughout the exam
Using the following information, do the conversions shown below, showing all work:
1 ft = 12 inches 1 pound = 16 oz 1 gallon = 4 quarts
1 mile = 5280 feet 1 ton = 2000 pounds 1 quart = 2 pints
kilo (= 1000) 1/100) milli (= 1/1000) deci (= 1/10) centi (=
1. 24.6 grams = ? kg
2. 6.3 ft = ? inches
1. 24.6 grams x 1 kg / 1000 g = 0.0246 kg
2. 6.3 ft x 12 in / 1 ft = 75.6 inches
please always use the correct units in your final answer
Question 3
Click this link to access the Periodic Table. This may be helpful throughout the exam.
Do the conversions shown below, showing all work:
1. 28oC = ? oK 2. 158oF = ? oC 3. 343oK = ? oF
1. 28oC + 273 = 301 oK oC → oK (make larger)
+273
2. 158oF - 32 ÷ 1.8 = 70 oC oF → oC (make smaller)
-32 ÷1.8
3. 343oK - 273 = 70 oC x 1.8 + 32 = 158 oF oK → oC → oF
Question 4 Click this link to access the Periodic Table. This may be helpful throughout the exam.
Be sure to show the correct number of significant figures in each calculation.
1. Show the calculation of the mass of a 18.6 ml sample of freon with density of 1.49 g/ml
2. Show the calculation of the density of crude oil if 26.3 g occupies 30.5 ml.
1. M = D x V = 1.49 x 18.6 = 27.7 g
2. D = M / V = 26.3 / 30.5 = 0.862 g/ml
Question 5 Click this link to access the Periodic Table. This may be helpful throughout the exam.
1. 3.0600 contains ? significant figures.
[Show More]