Health Care > EXAM > CITI Training Test Prep | 134 Questions with 100% Correct Answers (All)
CITI Training Test Prep | 134 Questions with 100% Correct Answers
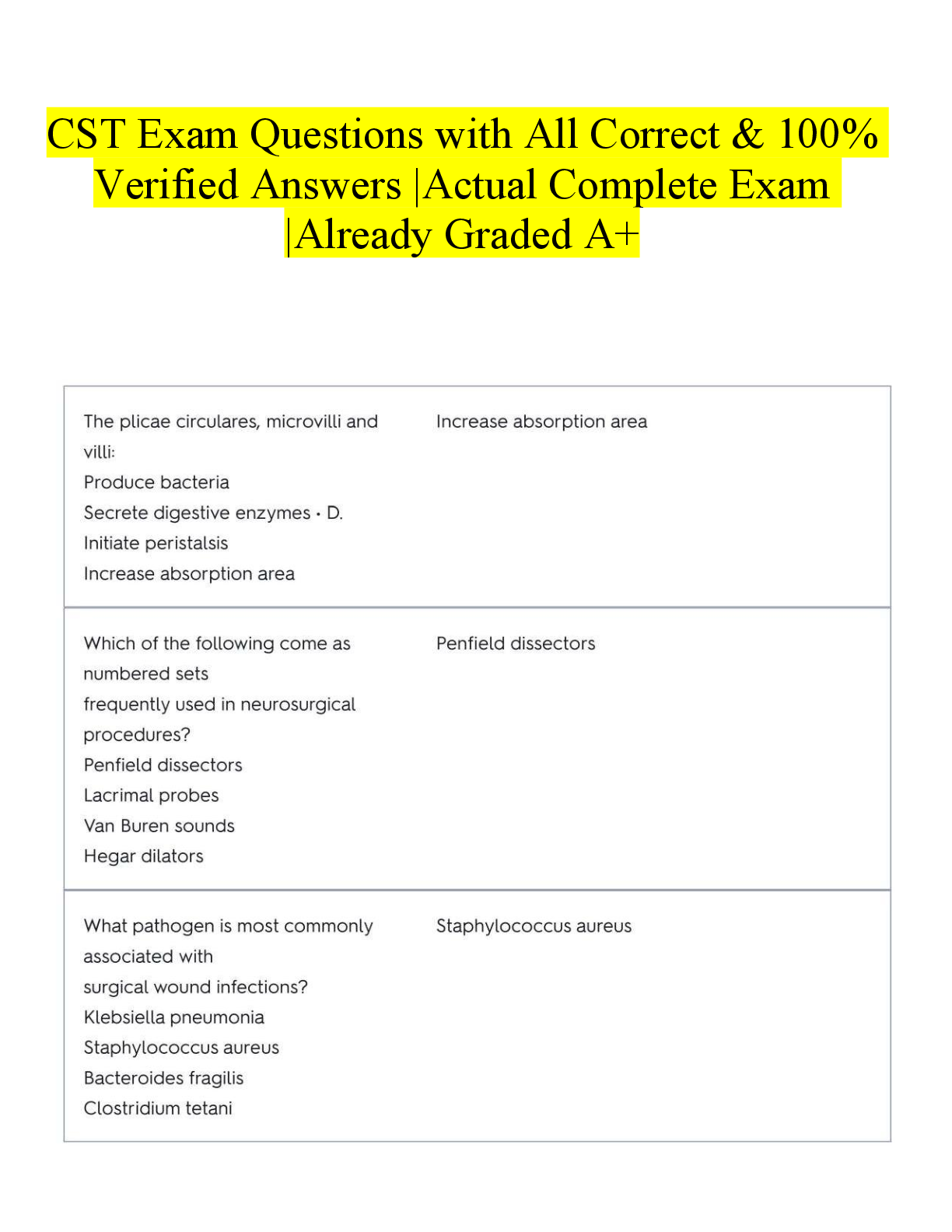
Document Content and Description Below
What must you file before conducting human clinical trials with an experimental drug? - IND application (Form FDA 1571) During the clinical development phase of the IND process, what must sponsors do? ... - Maintain current IND application by amending IND with new Form FDA 1571 and providing FDA with: safety updates copies of new protocols FDA 1572 Annual Progress reports this document notifies FDA of relevant changes in investigators conducting clinical trials under the IND. - Form FDA 1572 What is the timeline of drug development? - Preclinical trials, IND Submission, Clinical Development (Phase I-III), NDA submission, Marketing (Phase IV) When does a sponsor submit the IND? - Prior to clinical development phases (human trials). *30 day process. What is the NDA? - New Drug Application, submitted prior to Phase IV marketing phase. How long does the NDA submission take? [Show More]
Last updated: 3 years ago
Preview 1 out of 14 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Also available in bundle (1)
Click Below to Access Bundle(s)

CITI Training COMPLETE Compilation BUNDLE!!!
CITI Training COMPLETE Compilation BUNDLE!!!
By Tiara 3 years ago
$30.5
19
Reviews( 0 )
$10.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Oct 03, 2022
Number of pages
14
Written in
All
Additional information
This document has been written for:
Uploaded
Oct 03, 2022
Downloads
0
Views
79