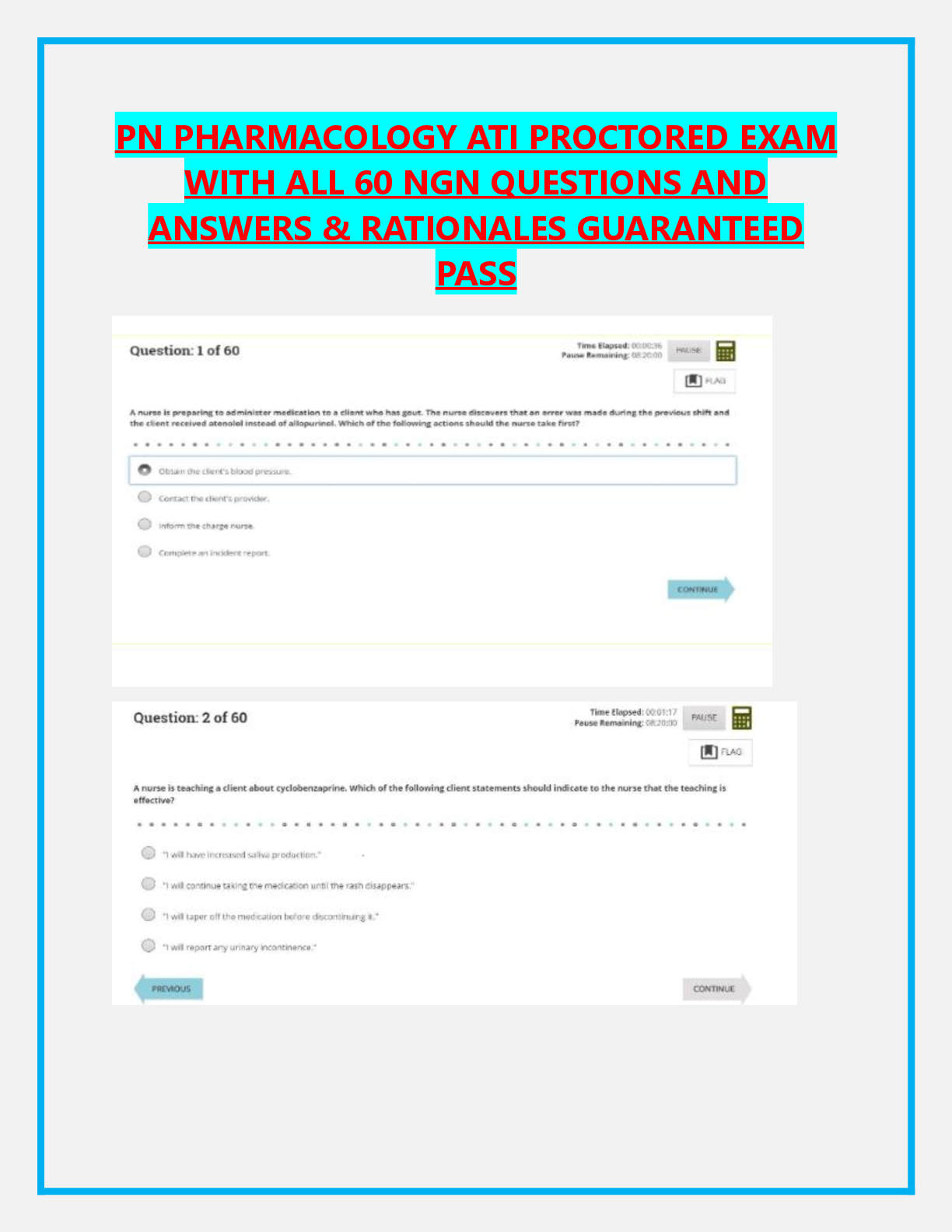
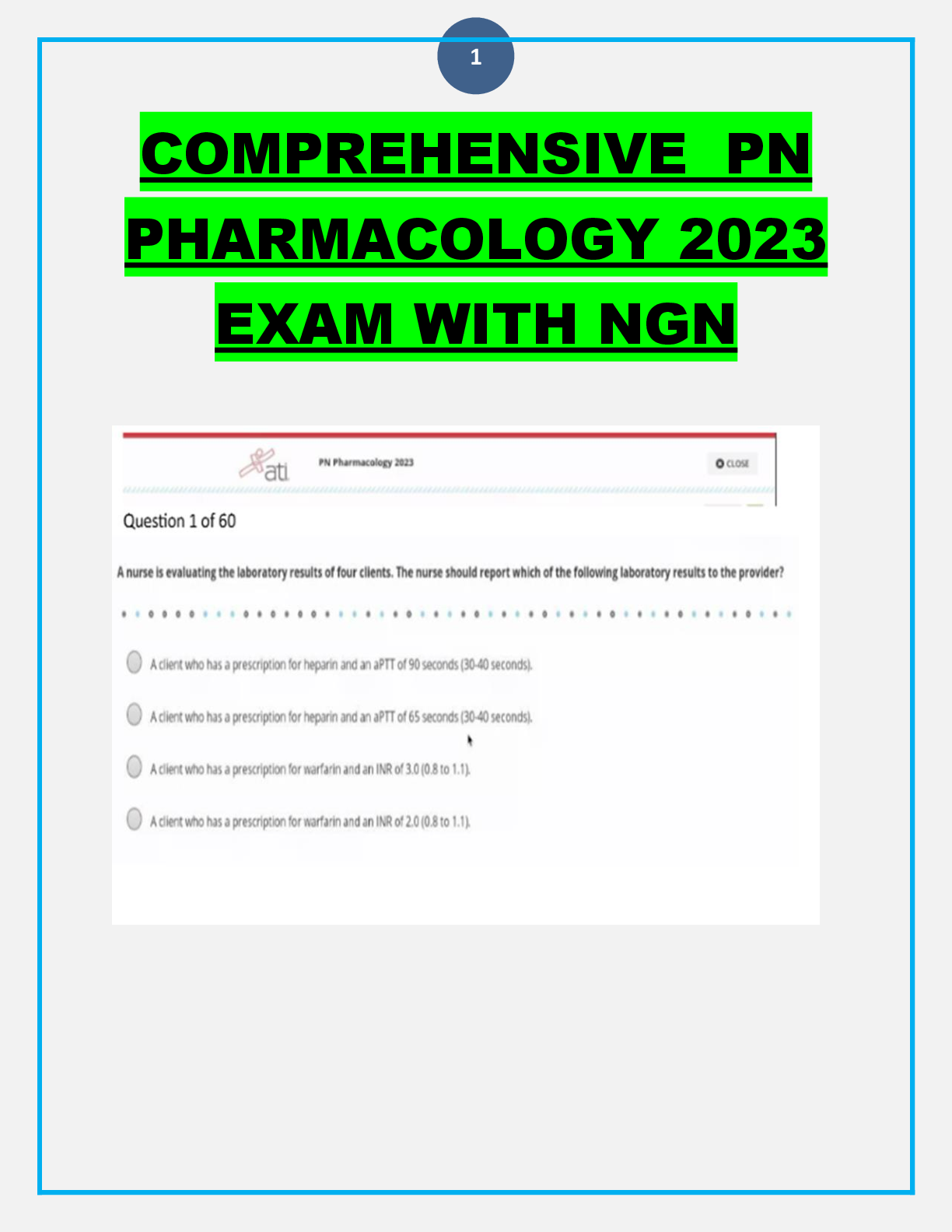
Compounding: - ANSWER Preparing/mixing a drug as a result of a prescription, and in anticipation of a prescription based on regularly observed prescribing patterns
Direct Supervision: - ANSWER A licensed pharmacist is
...
Compounding: - ANSWER Preparing/mixing a drug as a result of a prescription, and in anticipation of a prescription based on regularly observed prescribing patterns
Direct Supervision: - ANSWER A licensed pharmacist is physically available on-site in an area where pharmacy services are provided, to supervise the practice of pharmacy
Dispensing: - ANSWER Procedure resulting in receipt of a drug by a patient
Distribute: - ANSWER Sale or delivery of a drug to another licensee or registrant (NOT to a patient)
Pharmaceutical Care - ANSWER Provision of a drug regimen to achieve definite therapeutic outcomes
Pharmacy Technician Trainee: - ANSWER Individual engaged in a Board approved technician training program
Registered Pharmacy Technician: - ANSWER Technician that is registered with the Board
Practice of Pharmacy: - ANSWER Providing pharmaceutical care
Compounding and dispensing
Monitoring prescriptions and providing information
Acting within a Therapeutic Management Contract
Administering Vaccinations
Providing Drug Therapy Management
Pharmacy Intern: - ANSWER Registered with the Board of Pharmacy
Must practice under the direct supervision of a pharmacist
Dispensing of Prescription Drugs by Physicians, Dentists, or Podiatrists - ANSWER DISCOURAGED
Must have dispensing permit from licensing Board
Should only be done if a pharmacy is NOT conveniently available to the patient
Must label the drug under the rules of the Pharmacy Act
Must follow the Poison Prevention Packaging Act
Must perform biennial inventory of drugs stocked
Board of Pharmacy - ANSWER 2 Consumer Members
10 Ten Pharmacists
2 from chain pharmacy
2 from independent retail pharmacy
2 from acute-care hospitals
1 from long-term care facility
1 from home infusion/home care service
2 pharmacists at large
Reciprocity of Pharmacist Licenses - ANSWER Pharmacist in good standing and licensed by examination (NAPLEX) in another jurisdiction may apply for a Maryland pharmacist license without re-taking the NAPLEX exam
Applicant must take the Maryland MPJE
Pharmacist License Term and Renewal - ANSWER Must complete 30 hours of approved CE
within the prior 2 year period
Pharmacist license must be renewed every 2 years
All ACPE approved programs are approved in Maryland
Display of Licenses - ANSWER Pharmacist must conspicuously display license at place of business (employment)
Grounds for Discipline - ANSWER Providing professional services while under the influence of controlled substances or alcohol
Pays a prescriber to refer patients to the pharmacy (kick back)
Provides prescribers with prescription blanks bearing the name or address of the pharmacy
Aids in the unauthorized practice of pharmacy
Prepares secret/coded prescriptions
Fraudulently obtains license
Fraudulently collects a fee
Fraudulently advertises
Accepts employment from a prescriber who owns a pharmacy or joins with a prescriber to own a pharmacy
Advertises or claims professional SUPERIORITY
Is physically or mentally incompetent or is unable to safely practice pharmacy
Discloses confidential patient information without authorization (HIPAA violation)
Fails to offer patient counseling or to maintain patient history for new Medicaid prescriptions
Discriminates against a patient who is HIV positive
Is convicted/pleads guilty/pleads nolo contendre to a:
Felony
Crime involving dishonesty (moral turpitude)
Violation of Pharmacy Act or Board Regulation
Discipline by another state
Separate Pharmacy Permit for Separate Pharmacies - ANSWER A separate pharmacy permit is required for each separate geographic location
Separate permit is NOT needed for multiple pharmacies within one hospital or hospital complex
Required Standards/Rules for
Operation of a Pharmacy - ANSWER Must be supervised by a Pharmacist at all times it is open AND must have a PIC
Must provide complete pharmacy services
Not just "fast movers"
May NOT interfere with a patient's freedom to choose a pharmacy
Must maintain adequate personnel, technology, reference texts and equipment as specified by the Board
Must keep all prescription orders for at least 5 years from the date of the last refill
If a prescription log is kept which is signed by each patient picking up a prescription, the log must NOT show the patient's social security number, diagnosis, drug, etc. if the log can be viewed by other patients picking up prescriptions
Must provide information to patients on how to resolve incorrectly filled prescriptions
May post a sign
OR
May include written notice with the prescription
Display and Renewal of Pharmacy Permit - ANSWER expire on May 31st every 2 years
Permit must be conspicuously displayed in the pharmacy
Sterile Compounding Permits - ANSWER New in 2014
Products must be prepared under USP §797 standards
Does not require a patient specific prescription
Permit expires on May 31st of the next even-numbered year
Pharmacy must be inspected by the Board
Permit holder must report adverse events to the Board within 5 calendar days
Must also register with the FDA if DISTRIBUTING the compounded product
Refusal to Fill or Refill a Prescription - ANSWER A pharmacist may refuse to fill or refill a prescription based upon professional/scientific judgment
If a pharmacist refuses to fill or refill a prescription, the pharmacist shall notify the prescriber within 72 hours of the refusal
Drug Information and Pricing - ANSWER Only a pharmacist or an intern under the supervision of a pharmacist may provide information to a patient or a health care practitioner concerning drug therapy, side effects, use, etc.
If a patient requests, in person or by telephone, the current price for a prescription drug, the pharmacist shall provide the price
Dating and Time Limit to Fill Prescriptions - ANSWER Prescriber shall indicate the date of ISSUANCE on all prescriptions
A prescription shall not be filled after 120 days of issuance unless the prescriber consents/authorizes
Generic Substitution - ANSWER Generic substitution rules apply only if:
Prescriber orders the drug by its brand name
An FDA approved generic drug is available
The prescriber does NOT prohibit substitution
The prescription is NOT reimbursed by a third-party payer
If substitution rules apply:
A pharmacist shall inform the patient of the availability and cost of the generic equivalent drug
A pharmacist may substitute an FDA approved generic equivalent drug (Orange Book) that is less expensive than the brand name drug (Purple Book for Biosimilars)
The pharmacist shall notify the patient in writing of the substitution
The pharmacist shall record on the prescription blank the manufacturer or distributor of the generic drug dispensed
Labels of dispensed drugs must contain - ANSWER Date of filling
Expiration date of the lesser of 1 year from date of dispensing or manufacturer's expiration date
Special handling or storage requirements
Name and strength of the drug
Must contain same name as used by the prescriber on the prescription (avoid misbranding)
If a generic drug is substituted, must also add generic name (to avoid misbranding) and the name of the manufacturer or distributor of the drug
Patient alteration of label prohibited - ANSWER So long as any of the original drug remains in the vial/container, the patient is prohibited from altering, defacing, or removing the label of the prescription
A pharmacist may refill a prescription
without refill authorization IF: - ANSWER The drug is essential to the maintenance of life and to the continuation of therapy in a chronic condition
The pharmacist believes it would be detrimental to the patient's health if the drug was not provided
The pharmacist attempts to obtain
authorization but is unsuccessful
The drug is NOT a controlled substance
If a pharmacist provides an emergency
refill without authorization: - ANSWER Must record date, quantity, and sign or initial the record
May provide only 1 refill of a 14-day supply (or unit-of-use)
Must notify the prescriber within 72 hours
If a state of emergency is declared, a pharmacist may refill without authorization IF: - ANSWER The pharmacist is unable to obtain authorization
The drug is not a controlled substance
The quantity does not exceed a 14-day supply (or unit-of-use)
Pharmacist must notify prescriber within 7 days
Drug Information and Records (OBRA 90)
requirement - ANSWER Pharmacist or Pharmacist's designee must offer
to counsel medical assistance recipients on each new prescription
May be done face-to-face
OR
2 of the following:
Posted sign
Note affixed to prescription
Note affixed to bag
Communication by telephone
Only applies to Medicaid patients with new prescriptions (should do for all patients)
Pharmacist shall attempt to obtain medical history from the patient
Name, address, telephone number, date of birth, and gender
History of disease states, allergies, drug reactions, and drugs taken
Shall add pharmacist comments
Administration of Vaccines - ANSWER A Pharmacist may administer INFLUENZA vaccine to patients at least 9 years of age
Pharmacist must report vaccination to the ImmuNet Program
If vaccination was administered pursuant to prescription, pharmacist must attempt to inform prescriber
A pharmacist may administer any vaccination listed in the Centers for Disease Control and Prevention Recommended Immunization Schedule
The patient must be at least 11 years of age, but under the age of 18 years
The patient must have a prescription
The pharmacist must inform prescribing physician AND the patient's primary care physician that the vaccine was given
The pharmacist must report to the ImmuNet Program
A pharmacist may administer to an adult (over 18 years of age) any vaccination listed in the Centers for Disease Control and Prevention Recommended Immunization Schedule or Centers for Disease Control and Prevention's Health Information for International Travel
May be administered under a written vaccine specific protocol without a prescription
Pharmacist shall report the vaccination to the ImmuNet Program
If the vaccine was administered pursuant to a prescription, must attempt to notify the prescriber and the patient's primary care physician
Remote Automated Medication Systems - ANSWER Robotic machine located at a nursing home or clinic that does NOT have an on-site pharmacy
Pharmacist operates the system remotely using a computer
The pharmacist shall review all drug orders before they are entered into the machine
A starter dose may be given without a pharmacist's prior review IF the pharmacist does review the order within 24 hours of the delivery of the starter dose
The machine must: - ANSWER Have bar-code technology
Have electronic reporting capability of all persons accessing the machine and all medication removed from the machine
Provide a picture of the drug or a written description of the drug
Operate under a quality assurance program with policies and procedures
Drug Therapy Management Contracts (Collaborative Care Agreement) - ANSWER Written agreement, initiated by a physician, between a physician, pharmacist, and patient for the pharmacist to manage the patient's drug therapy
A contract is NOT required in a hospital setting
Pharmacist must have PharmD or other training as approved by the Board
The protocol may allow a pharmacist to:
Modify or discontinue but NOT INITIATE drug therapy
Order laboratory tests
Other patient care management
Contracts last 1 year unless renewed by all parties
Pharmacist technicians must be REGISTERED with the Board - ANSWER Technician trainees do not register
Pharmacy students do not register during school or for 10 months after graduation
Expiration & Renewal of Technician Registration - ANSWER Registration is valid for 2 years
To renew, technician must meet continuing education requirements set by the Board (20 hours every 2 years
Qualifications pharmacy technicians : - ANSWER Currently certified by a national program
OR
Be at least 17 years of age (16 ½ to start training)
High school graduate or GED or enrolled in high school
Complete a training program
At least 160 work hours
No longer than 6 months
Pass exam approved by the Board
Qualifications may be waived by the Board if the applicant will be working or has worked as a pharmacy technician in another state
Prohibited Acts/Required Acts of pharmacy technicians - ANSWER Technicians may NOT operate under a drug therapy management contract
Technicians may NOT administer influenza vaccine
Technicians may NOT delegate a task that was delegated to the technician
Technician may not commit an act similar to those prohibited to pharmacists
Registration must be displayed at place of business or be available for viewing
Must wear ID badge as a registered pharmacy technician
Qualifications Pharmacy Intern - ANSWER Must be currently enrolled and completed 1 year of professional education in an accredited pharmacy program
Must register with the Board before practicing under the supervision of a pharmacist
Must undergo a State criminal history records check
Must notify the Board within 7 days of conviction\plea to certain crimes
Pharmacist to Intern Ratio - ANSWER A pharmacist may not supervise more than 2 interns at one time
Authorized and Prohibited Acts pharmacy intern - ANSWER May not perform a final verification of a prescription
May not delegate a pharmacy act
May administer vaccinations
Must display or have available the registration in the pharmacy where working
Prescription Drug Repository Program - ANSWER Program for pharmacies to accept donated prescription drugs for disposal AND dispensing to needy patients
If the drugs are donated for dispensing:
Must be in original, sealed, tamper-evident unit dose packaging
Must have an expiration date equal to or greater than 90 days
Must not be adulterated or on a list of unacceptable drugs (
[Show More]