BioChemistry > QUESTIONS & ANSWERS > GIZMO: Polarity and Intermolecular Forces Lab sheet: Student Exploration: Polarity and Intermolecula (All)
GIZMO: Polarity and Intermolecular Forces Lab sheet: Student Exploration: Polarity and Intermolecular Forces
Document Content and Description Below
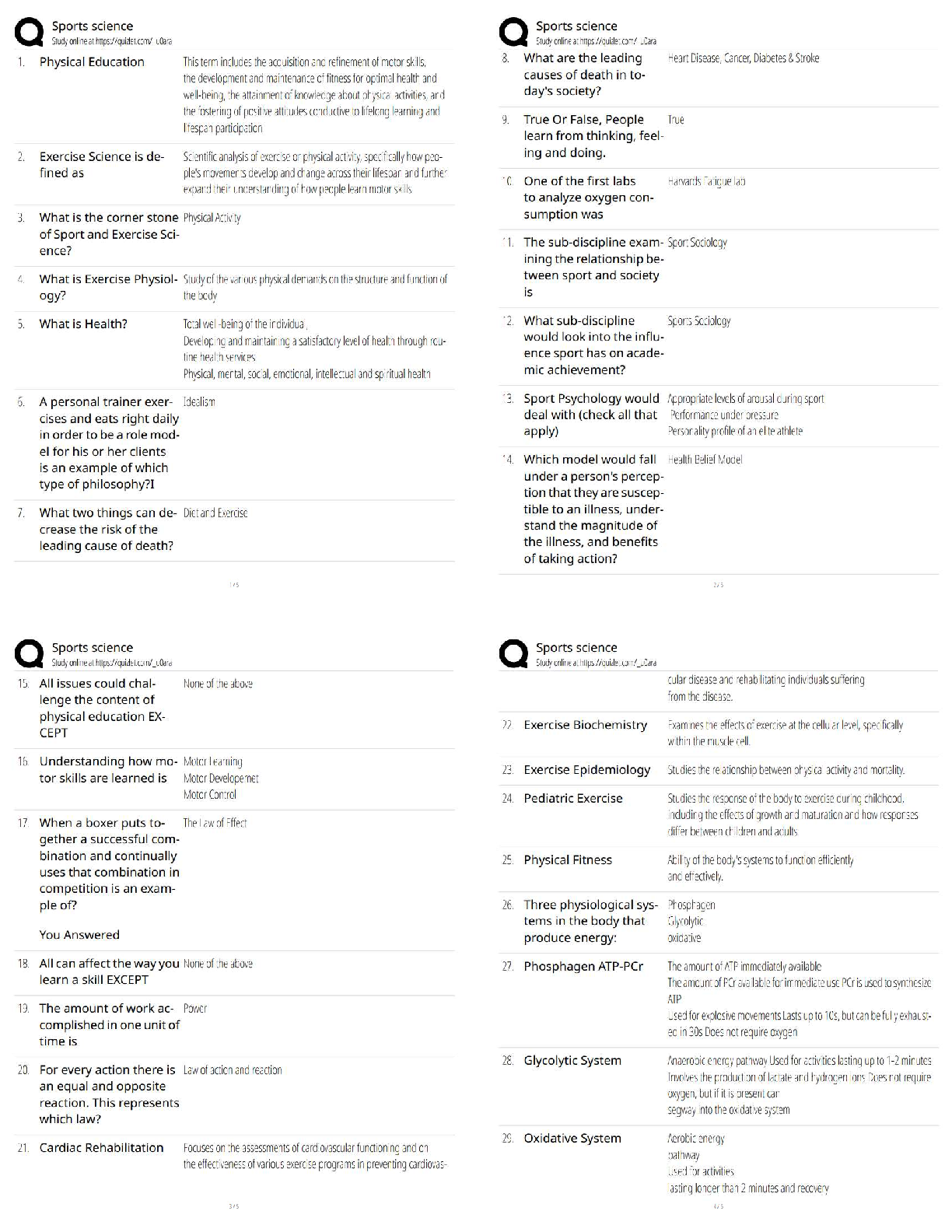
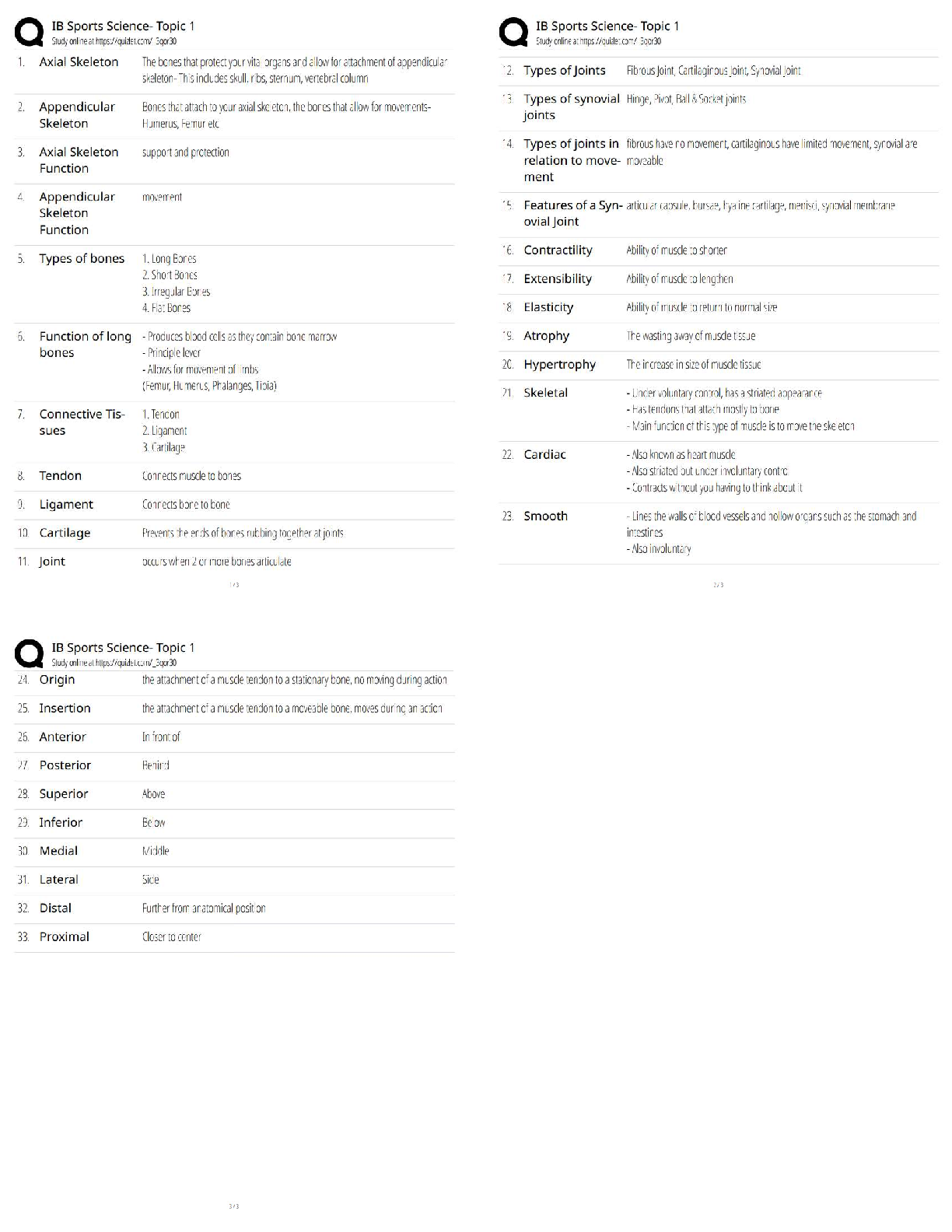
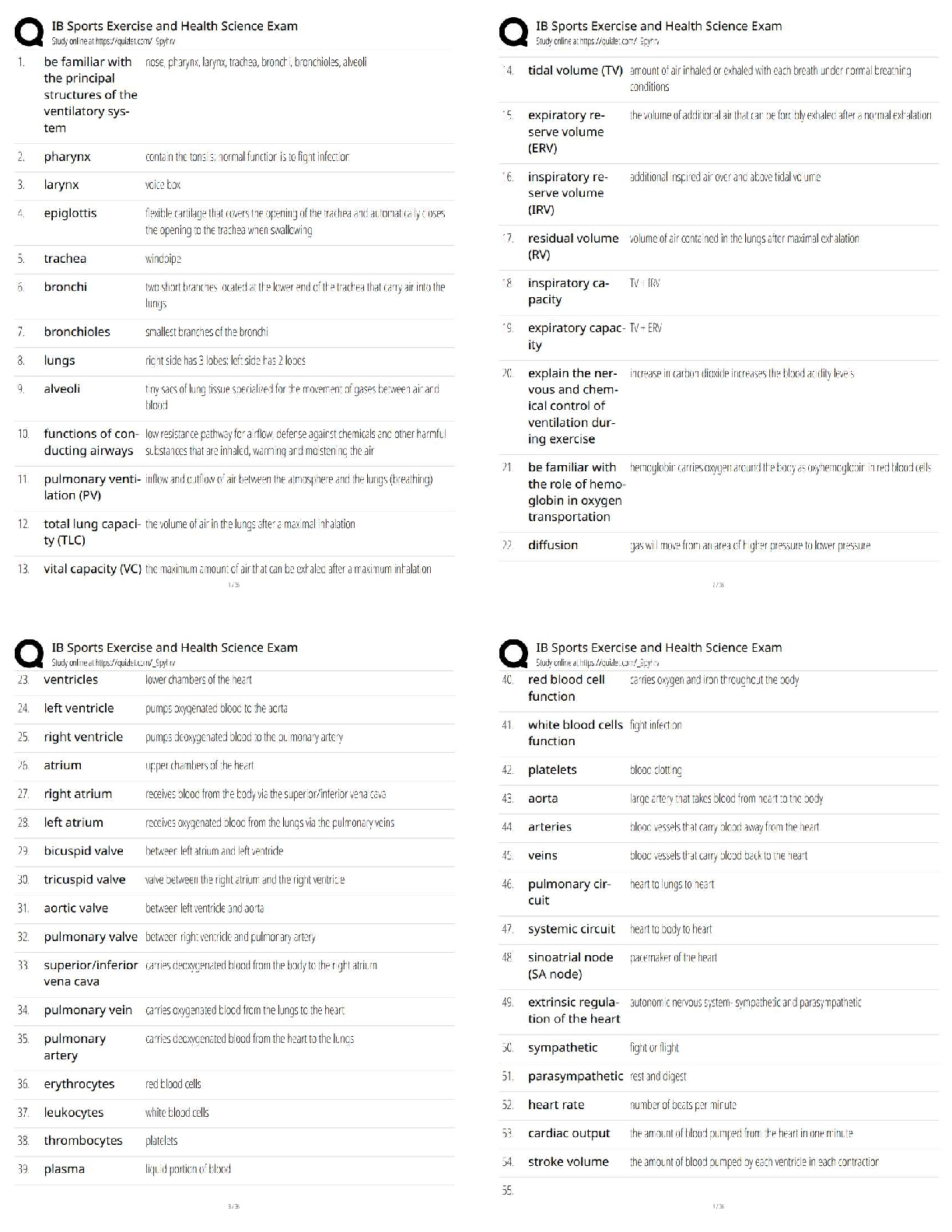
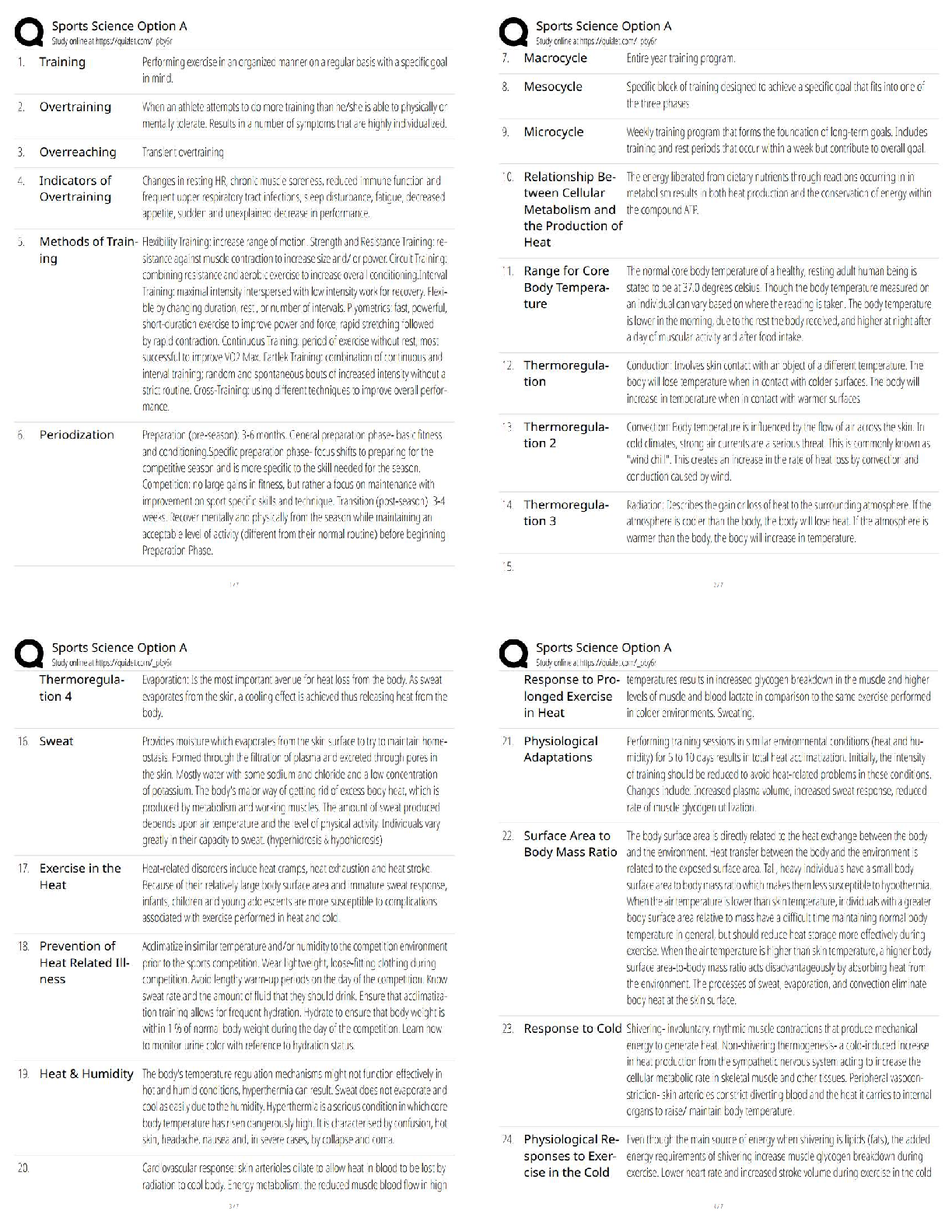
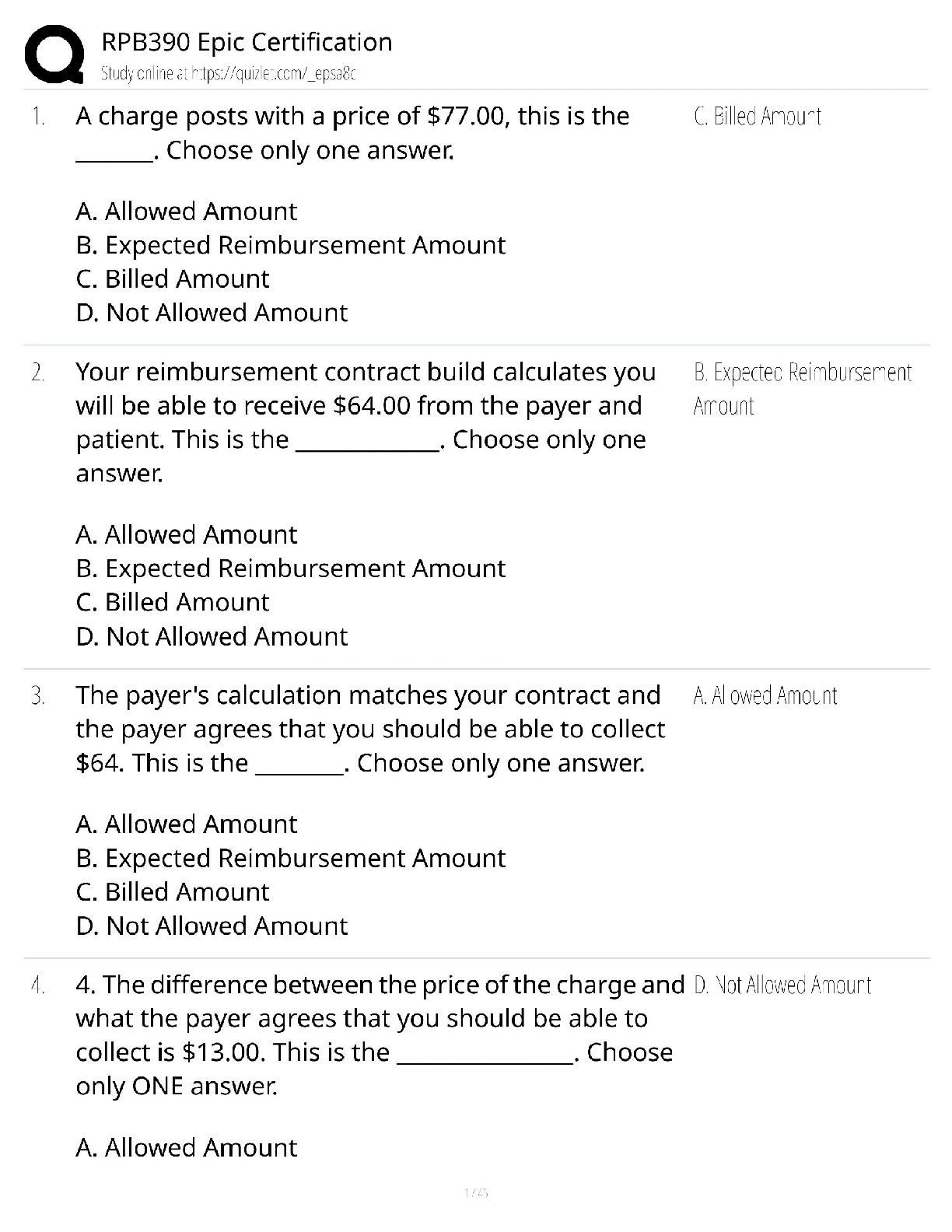
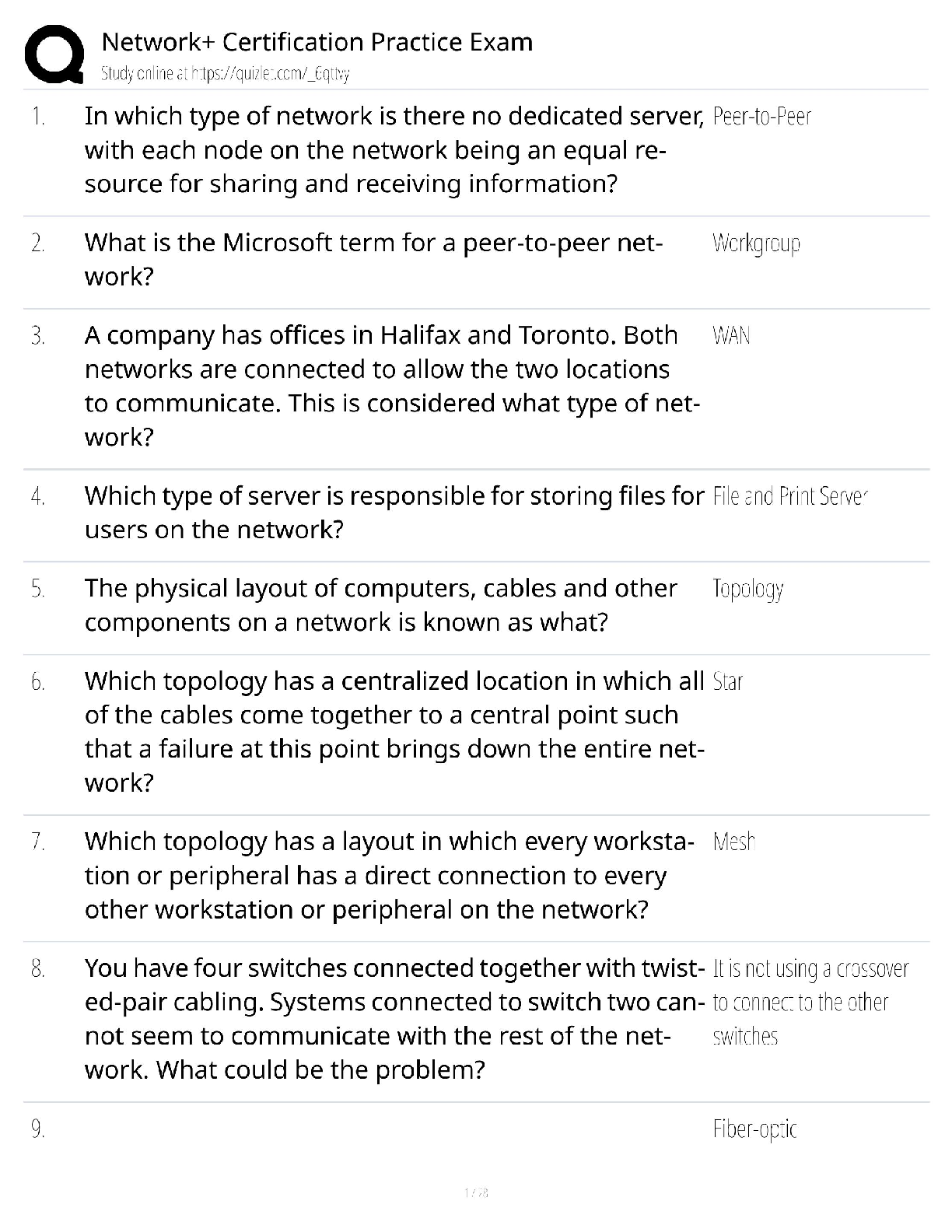
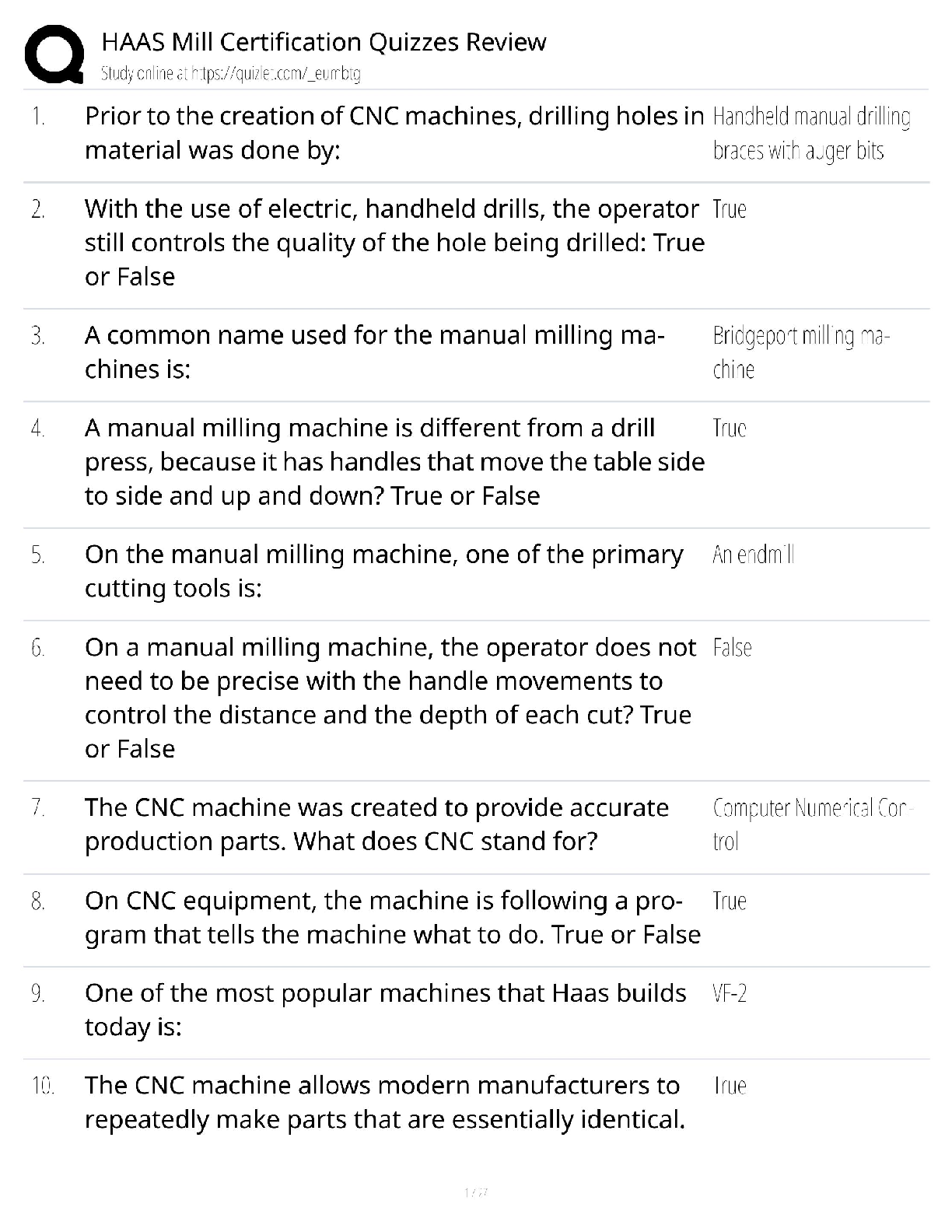
GIZMO: Polarity and Intermolecular Forces Lab sheet: Student Exploration: Polarity and Intermolecular Forces Polarity and Intermolecular Forces Lab sheet: Student Exploration: Polarity and Intermolec ... ular Forces Name: Lucas Vale Date: April 23 Total /65 Student Exploration: Polarity and Intermol ecular Forces Vocabulary: dipole, dipole-dipole force, dipole-induced dipole force, electronegativity, intermolecular force, ionic bond, London dispersion force, molecule, nonpolar, nonpolar covalent bond, partial charges, polar, polar covalent bond, valence electron Prior Knowledge Questions (Do these BEFORE using the Gizmo.) 1. A big bully is having a tug-of-war with a small child. There is a ball attached to the middle of the rope. Toward whom will the ball move? (1)The big bully 2. Two equally strong kids are having a tug-of-war. What do you expect to happen to the ball in this situation? (1) I expect the ball to not move and just stay in the middle Gizmo Warm-up Just like in a tug-of-war, atoms that are bonded to one another pull on the electrons they share. In the Polarity and Intermolecular Forces Gizmo, you will explore how these opposing forces relate to bond types and the forces between molecules. To begin, drag the Na (sodium) and Cl (chlorine) atoms into the simulation area. Turn on Show valence electrons. A valence electron is found in the outermost energy level of the atom. 1. Click Play ( ). What do you notice? (1) The chlorine atom takes the valence electron from the sodium atom. 2. Which atom seems to be pulling more on sodium’s one valence electron? (sodium or chlorine) (1) chlorine How do you know? (1)I know because the valence electron is now in chlorines valence shell since it pulled the electron more than the sodium did, it now has a more negative charge. 3. What happens to the colors of the simulated atoms, and what does this indicate? (1) The sodium atom turned blue which indicates a positive charge and the chlorine atom turned red which indicates a negative charge. Drag the atoms/molecule down to the bottom to remove them from the simulation area. Activity A: Bond polarity Get the Gizmo ready: ● On the BOND POLARITY tab, click Reset ( ). ● Drag the atoms out of the simulation area. Introduction: A neutral atom has the same number of protons as electrons. Atoms that gain electrons become negatively charged, while those that lose electrons become positive. A polar bond forms when shared electrons are pulled closer to one atom than another, causing the bonded atoms to become partially charged. In a nonpolar bond, electrons are shared equally. Question: What causes bonds to be polar or nonpolar? 1. Observe: Select the Show polar molecule inset checkbox. The animation shows the probable location of electrons (orange dots) in a polar molecule. A. What do you notice about the distribution of the electrons? (1)On average the electrons are closer to one atom then the other. B. How does this electron distribution affect the charges of the bonded atoms? (1)The atom that has more electrons distributed to it will have a more negative charge then the other atom. 2. Observe: Turn on the Show nonpolar molecule inset. A. How are the electrons in this molecule distributed? (1)The electrons are spread evenly across the atoms. B. Why do the bonded atoms remain neutral? (1)They remained neutral because the electrons were evenly shared/distributed, therefore there is no imbalance of electrons. 3. Experiment: Turn off Show polar molecule inset and Show nonpolar molecule inset. Check that Show valence electrons is turned on. Drag the Na and Cl atoms into the simulation area. Click Play. Note the colors. Red indicates a negative charge, while blue indicates a positive charge. A. What type of bond does it form? Drag the molecule to the three boxes to the right to see what type of bond it forms. When it is in the right box, it will say correct! (1) It will form an ionic bond B. Try several other metal/nonmetal combinations. What do you notice about the bonds that form? (1) They all form ionic bonds Ionic bonds are polar bonds that form between metal and nonmetal atoms. In this bond, valence electrons are transferred from a metal to a nonmetal. Drag each of these metal/nonmetal combinations into the Ionic bond bin on the upper right. (Activity A continued on next page) Activity A (continued from previous page) 4. Experiment: Now try forming bonds between different combinations of nonmetals. A. What do you notice? (1) They form both polar covalent and nonpolar covalent bonds. B. Are all of these bonds polar? Explain. (1) No they are also non polar. 5. Experiment: Turn on Show electronegativity. Electronegativity (EN) describes how strongly an atom attracts a pair of shared electrons. The higher the EN value, the greater the tendency of an atom to hold onto electrons in a bond and become negatively charged. Electronegativity difference is found by subtracting the EN value of one atom from another. Choose two nonmetals with a small (or no) EN difference between them. Click Play. What happens to the shared valence electrons in this bond? Look at the distance of shared electrons to each atom’s valence orbital. (1) They are the same difference from the both atoms as they move away from their original valence shell. When the shared electrons experience the same attraction from each atom, the result is a nonpolar covalent bond. Drag the bonded atoms to the Nonpolar covalent bond bin. 6. Experiment: Choose two nonmetals with a large electronegativity difference. Click Play. What happens to the shared valence electrons in this bond? Look at the distance of shared electrons to each atom’s valence orbital. (1) They are pulled much more to one of the atoms then the other. A bond in which the electrons are shared unequally is a polar covalent bond. Notice the small δ+ and δ– symbols, which indicate partial charges. Drag the resulting combination to the Polar covalent bond bin. 7. Classify: Use the Gizmo to categorize the remaining element combinations as forming either ionic, polar covalent, or nonpolar covalent bonds. Write each combination (formula) below its classification. (10) Ionic (EN difference > 1.67) eg. NaCl CaO, Na2O, CaCl2, MgCl2, KCl, NaCl, MgO, K2O Polar covalent (EN diff. < 1.67 and > 0.40) CO2, NH3, CCl4, H2O, OCl2, CO Nonpolar covalent (EN difference < 0.40) NCl3, O2, CH4,N2,Cl2,H2 Activity B: Molecular polarity Get the Gizmo ready: ● Select the MOLECULAR POLARITY tab. Introduction: Ionic bonds, like those found in NaCl, form crystalline solids. Covalent bonds, on the other hand, usually form discrete molecules. A polar molecule, while neutral overall, has a slight positive charge on one end and a slight negative charge on the other. Question: What determines the polarity of molecules? 1. Observe: Notice that the molecules containing polar covalent bonds are grouped together at lower left, and the molecules containing nonpolar covalent bonds are at lower right. Drag the H2O molecule into the simulation area. A. Are the individual bonds in this molecule polar or nonpolar? (1) polar B. Click Turn on electric field. What do you notice? (1) The atom rotates. The positively charged hydrogen atoms go towards the negative charge and the negatively charged oxygen atom goes to the positive charge. C. The electric field consists of a positively charged plate on one side and a negatively charged plate on the opposite side. Which side of the H2O molecule is attracted to the positive plate, and why do you think this attraction occurs? (2) The side with the oxygen atom is attracted to the positive plate. I think this occurs because the oxygen is negatively charged and opposites attract. D. Click Reverse field. Why does the H2O molecule rotate 180°? (1) It rotates because the hydrogen is attracted to the negative charged plate and the oxygen is attracted to the positive charged plate. A polar molecule, such as H2O, changes its orientation when placed in an electric field. The positive end of the molecule is attracted to the negative plate, while the negative end is attracted to the positive plate. Drag this molecule into the Polar bin. 2. Observe: Drag the CH4 molecule into the simulation area. A. Do you think this molecule will rotate in the electric field? Why or why not? (1) I do not think it will rotate in the electric field because it is non polar and the electrons are evenly distributed. B. Turn on the electric field. Is this molecule polar or nonpolar? (1) It is non polar. Drag the CH4 molecule into the appropriate bin. (Activity B continued on next page) Activity B (continued from previous page) 3. Classify: With the Gizmo, test and categorize the remaining molecules. Report your findings. (6) Polar molecules H2O, NH3, OCL2, CO, NCL3 Nonpolar molecules CCl4, CO2, H2, O2, N2, Cl2, CH4 Did the polarity of any of these molecules surprise you? Explain. (1) Yes, the polarity for NCL3, CCl4, and CO2 surprised me. This is because they went in the box opposite to what their bonds said that they were. 4. Explain: Drag the CCl4 molecule into the simulation area. A. Does this molecule contain polar or nonpolar bonds? (1) It has polar bonds. B. Turn on the electric field. Why do you think the molecule does not rotate in this field? (1) I think it does not rotate because it is actually non polar and is symmetrical so it is all distributed evenly. If a molecule is symmetrical, the effect of the partial charges on either side cancel out. In this case, even though it contains polar bonds, the molecule as a whole may be nonpolar. C. What other nonpolar molecule contains polar bonds? (1) CO2 5. Challenge: Find an example of a polar molecule that contains nonpolar bonds. Drag this molecule into the simulation area. A. Which molecule did you select? (1) NCl3 B. Turn on Show valence electrons. What do you notice at the very top of the nitrogen atom, and how does this feature explain why the molecule is polar? (1) At the very top of the nitrogen atom there is a negative charge, meaning it is polar because it is partially charged For the NH3 molecule, the lone pair of valence electrons shown at the top spread out, bending the chlorine atoms downward due to electron repulsion. As a result, the molecule is slightly polar. Activity C: Intermolecular forces Get the Gizmo ready: ● Select the INTERMOLECULAR FORCES tab. Introduction: The polarity of molecules give rise to the forces that act between them. These intermolecular forces, or IMFs, affect many physical properties including boiling point, solubility, viscosity, and surface tension. Question: How does polarity affect the forces between molecules? 1. Observe: Select the Show polar molecules inset checkbox. What do you notice? (1) An intermolecular bond is formed between the positive side of one polar molecule and the negative side of another. Each polar molecule is known as a dipole. The attraction between the positive end of one dipole and the negative end of another is called a dipole-dipole force. 2. Observe: Turn on the Show nonpolar molecules inset. What do you notice? (1) To form an intermolecular bond between non polar molecules, the molecules become temporarily polar. Even when the molecules are nonpolar, random variations in the distribution of electrons can cause parts of these molecules to become slightly charged. This imbalance leads to very tiny, short-lived attractions between molecules called London dispersion forces. 3. Experiment: Turn off Show polar molecule inset and Show nonpolar molecule inset. Drag two H2O molecules into the simulation area, and click Play. Click Pause ( ) when you see a bond form between molecules. Sketch the molecules, partial charges, and the bond between them in the space to the right. (2) Why is a hydrogen atom in one H2O molecule attracted to the oxygen atom in an adjacent H2O molecule? (1) Because hydrogen is positive and oxygen is negative and opposites attract. Drag the H2O-H2O molecule combination into the correct bin on the right. Which type of intermolecular force causes attraction between H2O molecules? (1) Dipole-Dipole (Activity C continued on next page) Activity C (continued from previous page) 4. Experiment: Drag two O2 molecules into the simulation area, but do not click Play yet. A. What force do you expect to see between O2 molecules? (1) I expect them to not have an attractive force. B. Click Play and observe. What do you notice? (1) They occasionally have a bond with another O2 molecule C. Drag the O2-O2 molecule combination into the correct bin on the right. Which type of intermolecular force causes attraction between O2 molecules? (1)London Dispersion 5. Experiment: Drag an O2 molecule and an H2O molecule into the simulation area. Click Play, and then click Pause when you see a bond form. A. What happens to the end of the O2 molecule that is closest to the positive end of the H2O molecule? (1) It shrinks and gets a positive charge or grows and gets a negative charge B. As the bond forms, does the polarity of the O2 molecule change? (1) yes the polarity changes C. Classify this combination of molecules. (It might take a few tries to get it right.) Which type of intermolecular force is acting here? (1) Dipole induced dipole When a polar molecule approaches a nonpolar molecule, the electron cloud of the nonpolar molecule may become distorted, causing the nonpolar molecule to become temporarily polar. For example, if the negative end of the polar molecule approaches the nonpolar molecule, the electron cloud of the nonpolar molecule will be repelled, causing a slight positive charge at that end of the nonpolar molecule. The resulting attractive force is called a dipole-induced dipole force. 6. Classify: Drag out different combinations of molecules in the Gizmo and categorize them. Give at least three examples of molecule combinations for each intermolecular force. (5) Dipole-dipole forces H2O-H2O, OCl2-NH3,CO-NCL3, CO-CO Dipole-induced dipole forces H2O-O2, CO-H2, CO-N2, CO-O2 London dispersion forces O2-O2, N2-N2, Cl2-Cl2, CO2-CO2, CO2-CH4 7. Summarize: Fill in the blanks to summarize the patterns you see. Dipole dipole forces arise between polar molecules.(1) london dispersion forces arise between nonpolar molecules.(1) dipole induced dipole forces arise between polar and nonpolar molecules. [Show More]
Last updated: 3 years ago
Preview 1 out of 7 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$10.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Jan 13, 2021
Number of pages
7
Written in
All
Additional information
This document has been written for:
Uploaded
Jan 13, 2021
Downloads
0
Views
118




.png)


.png)




.png)