Clinical Chemistry > QUESTIONS & ANSWERS > COORDINATING CLINICAL RESEARCH PRACTICE EXAM WITH CORRECT ANSWERS|GRADED A (All)
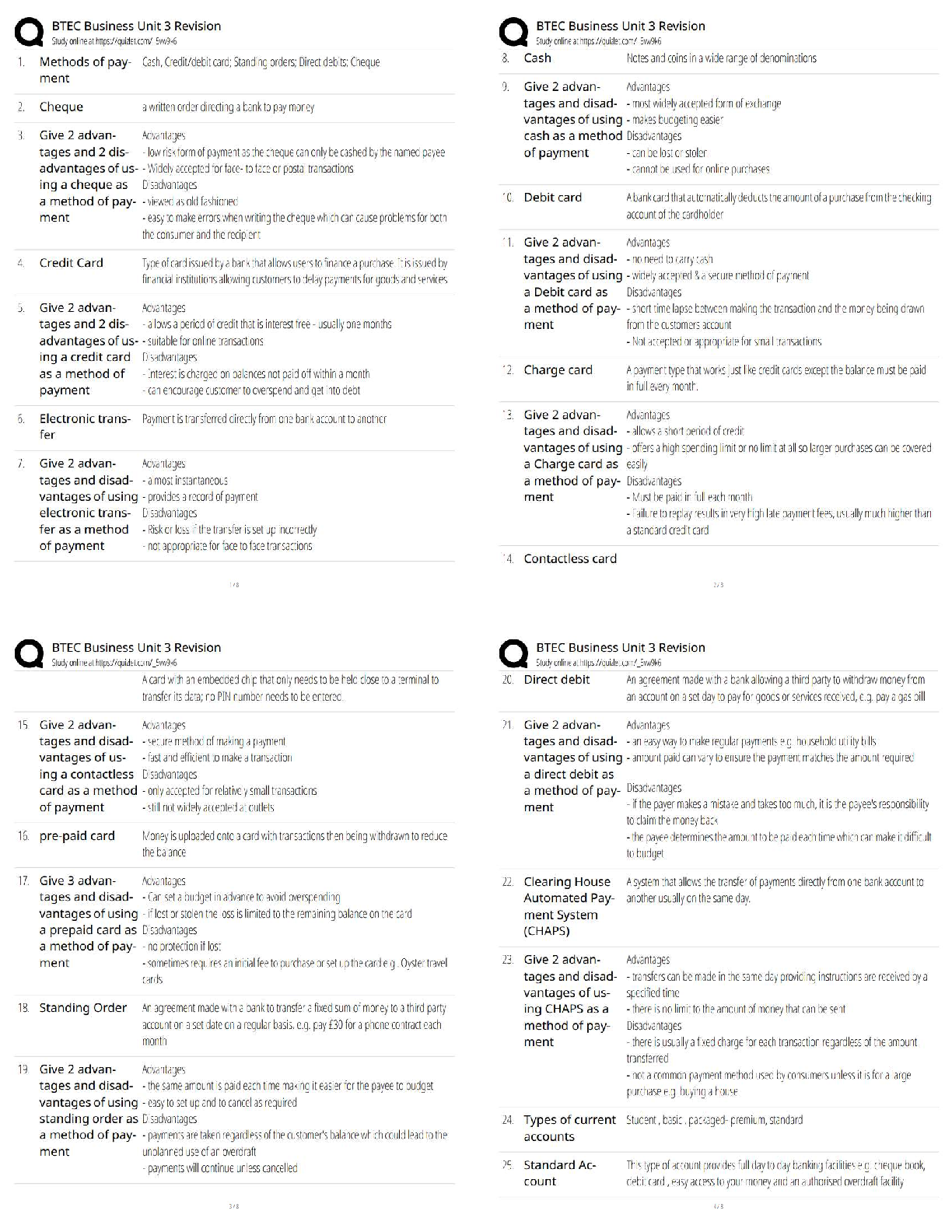
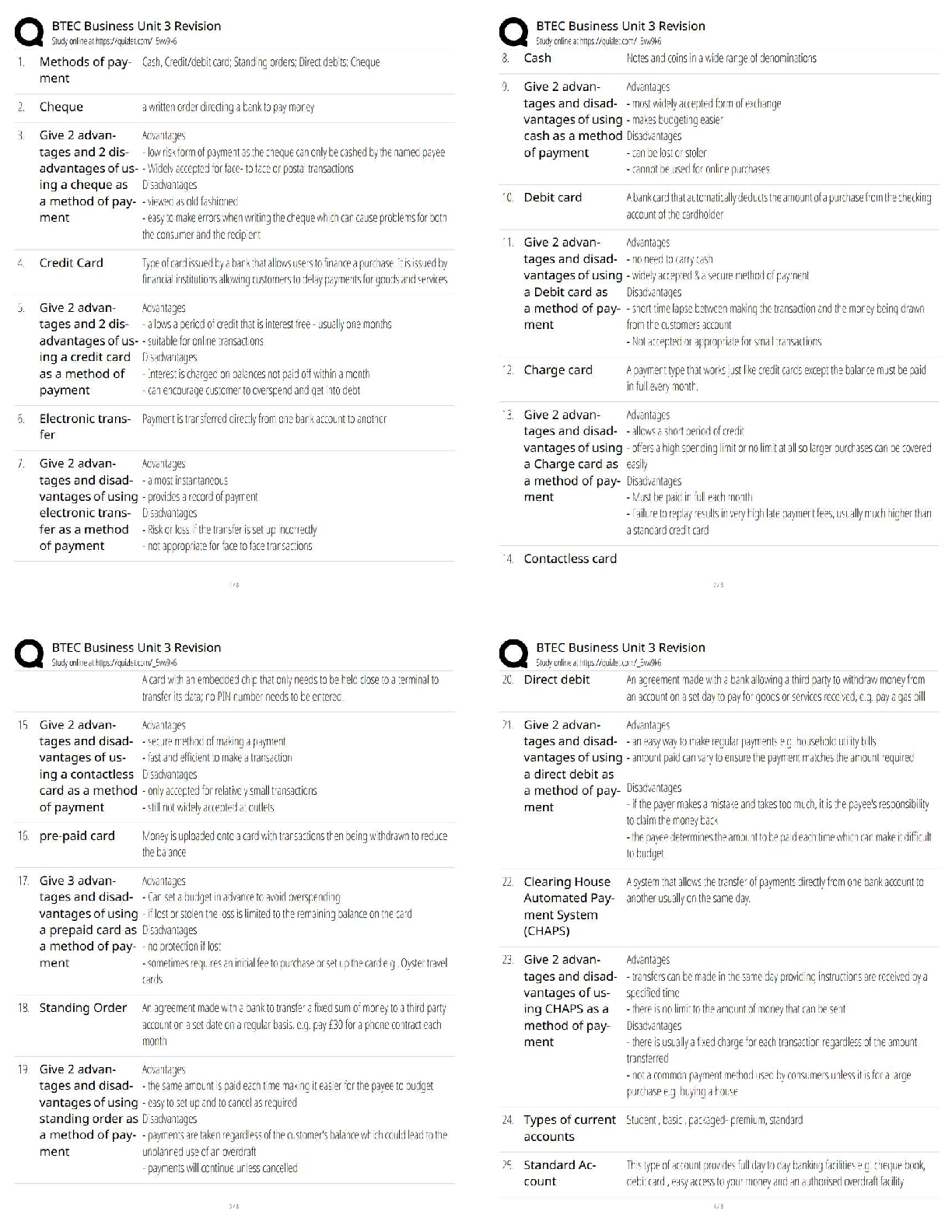
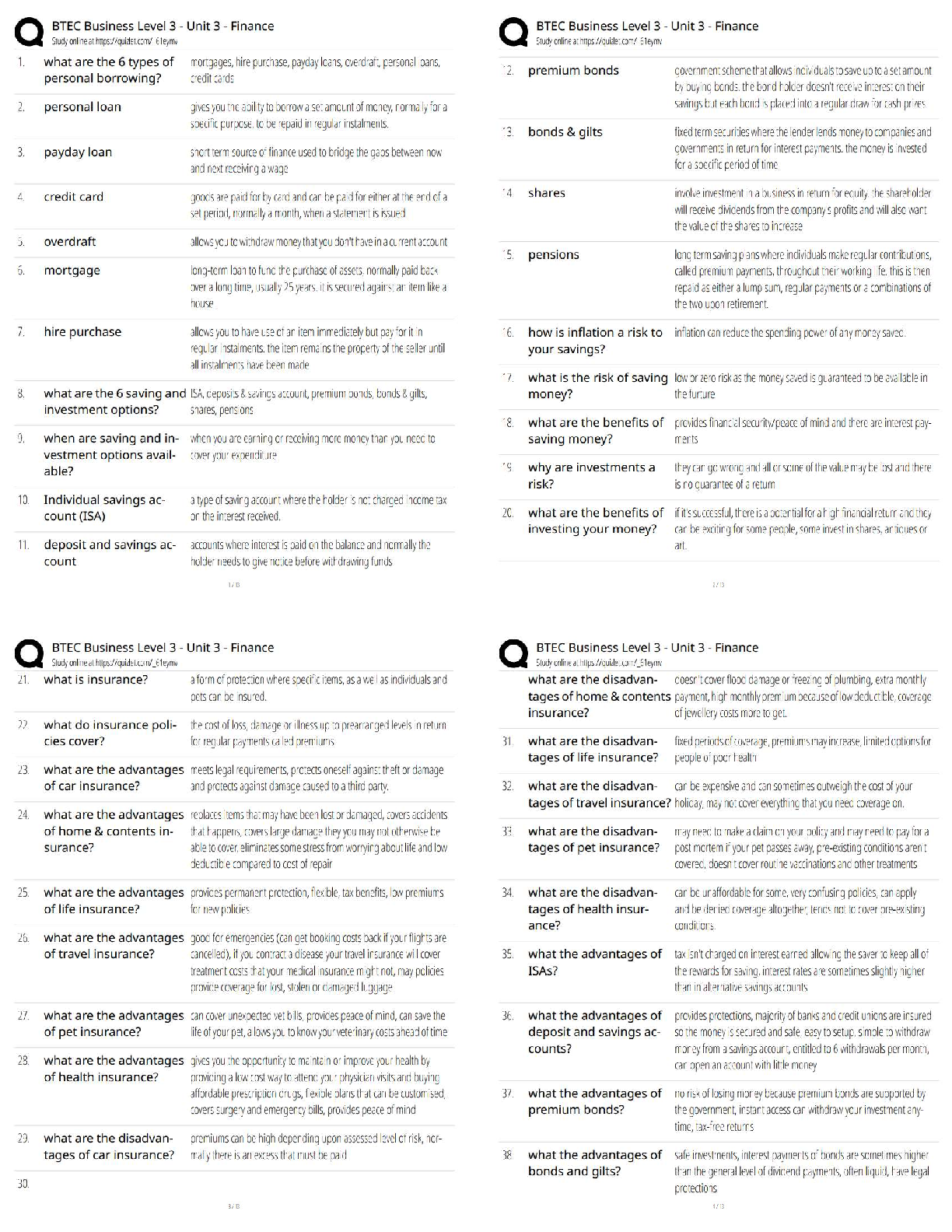
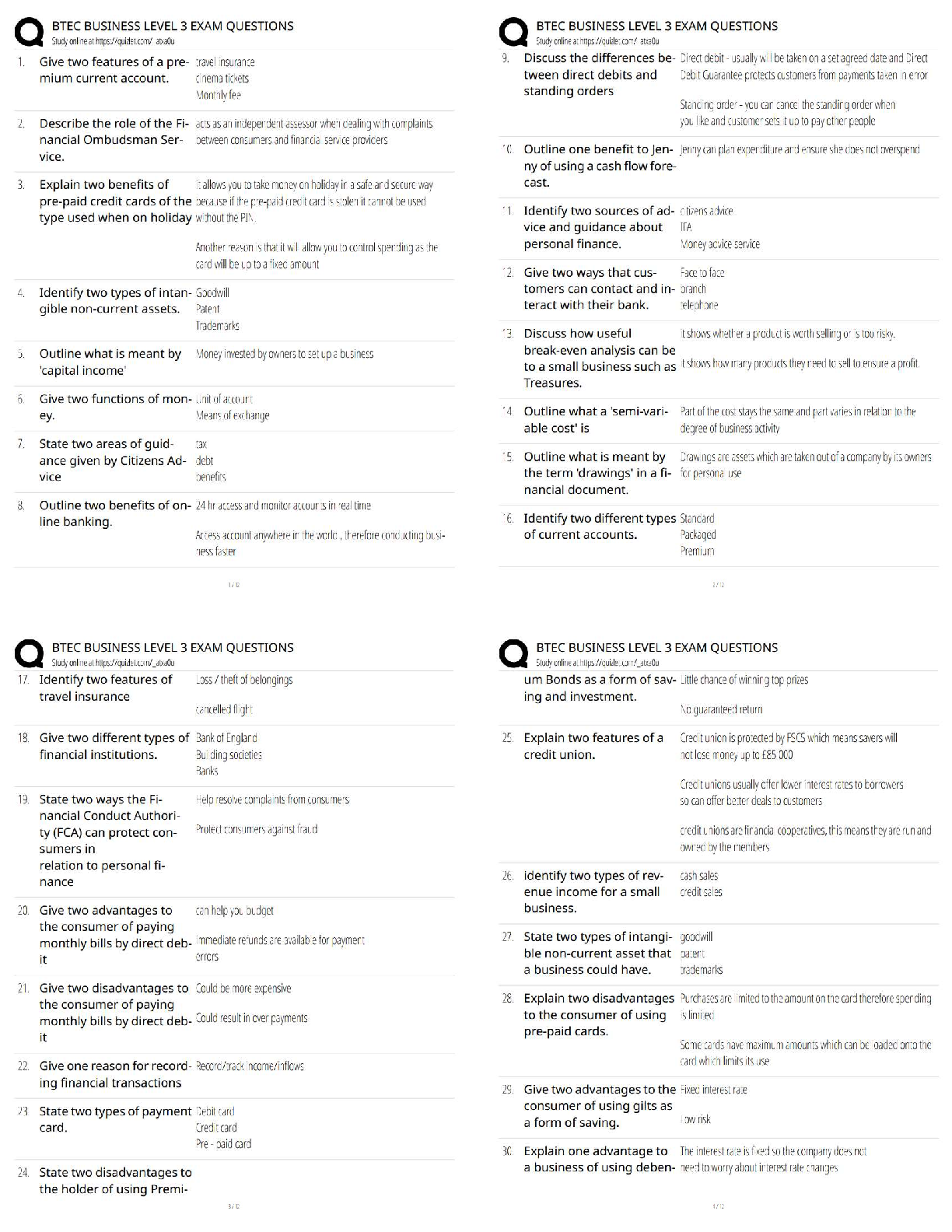
COORDINATING CLINICAL RESEARCH PRACTICE EXAM WITH CORRECT ANSWERS|GRADED A
Document Content and Description Below
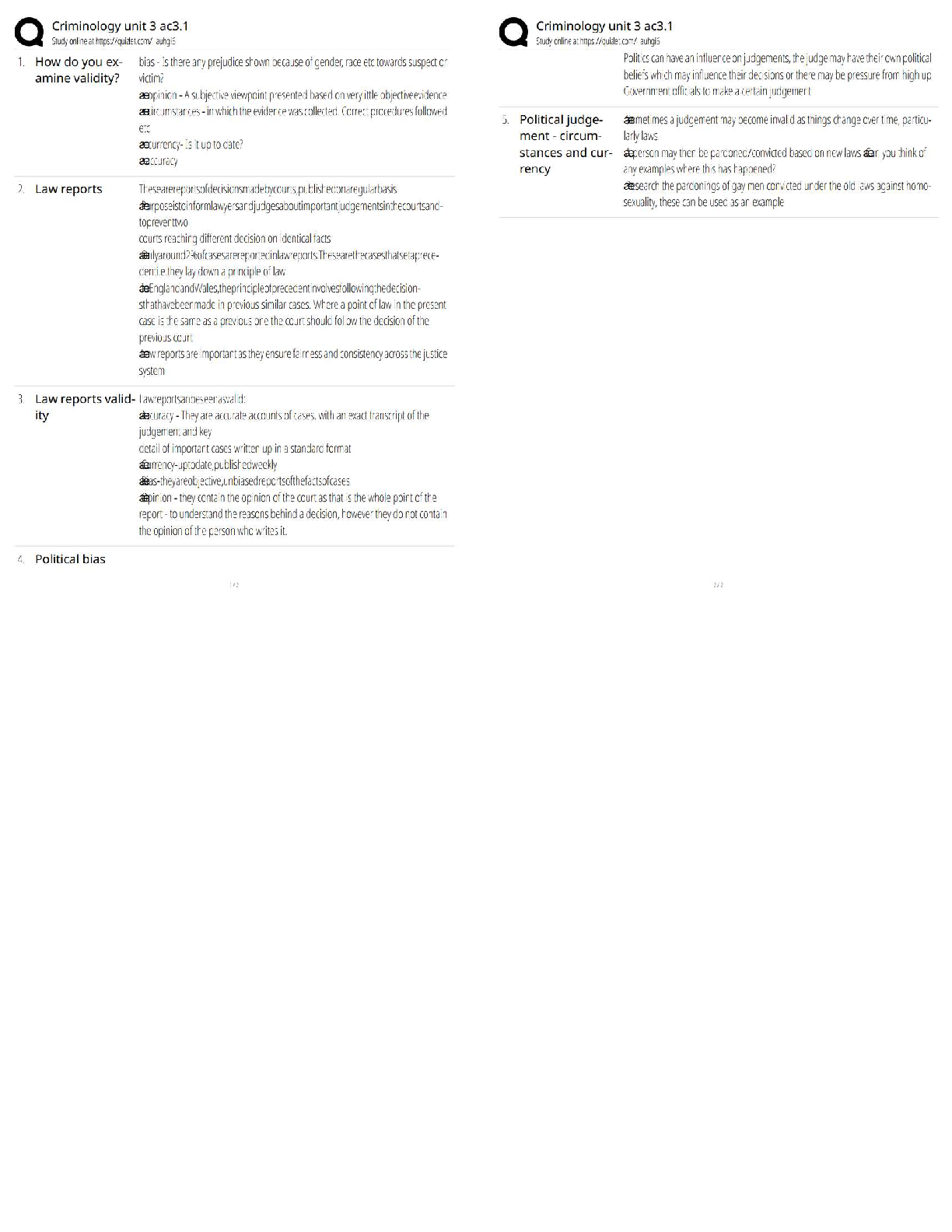
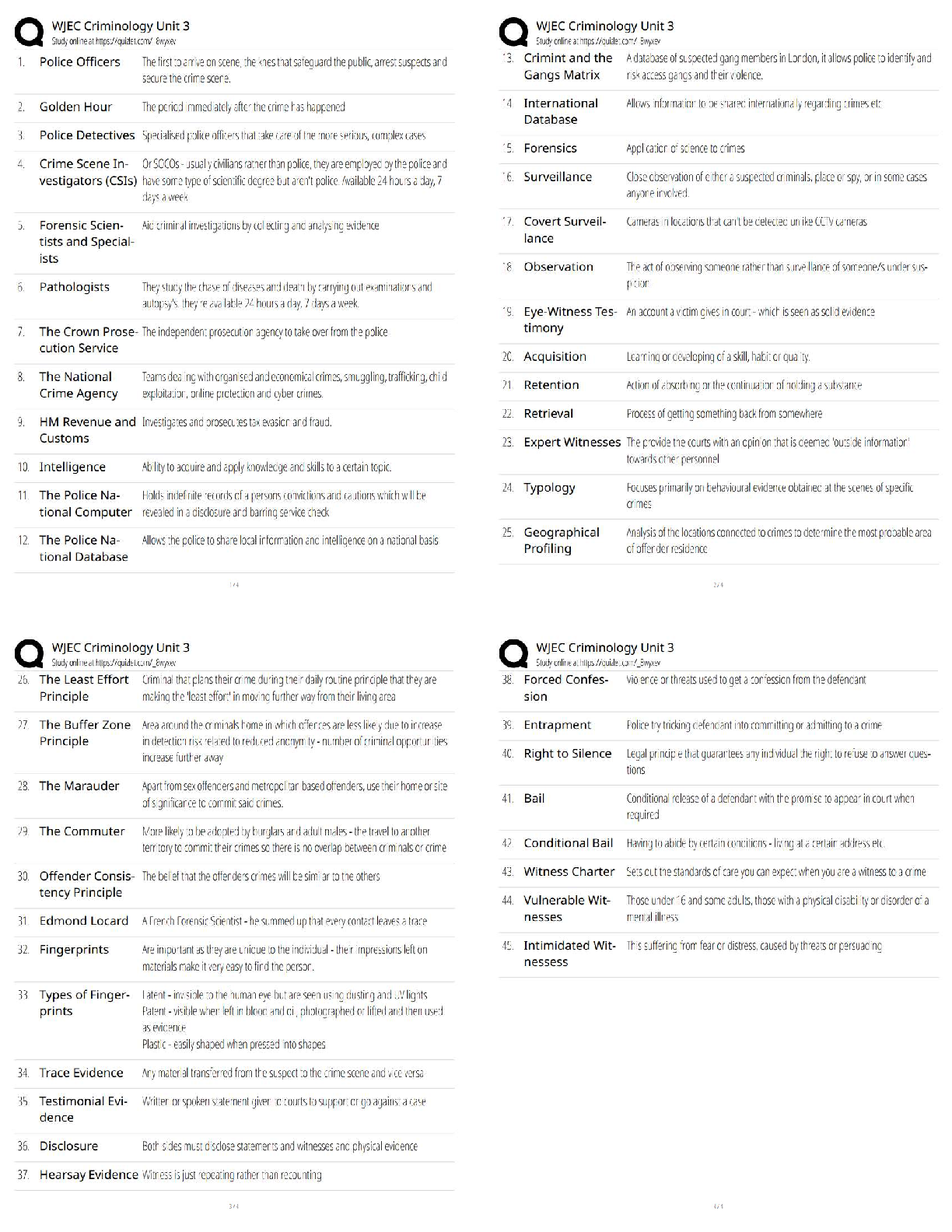
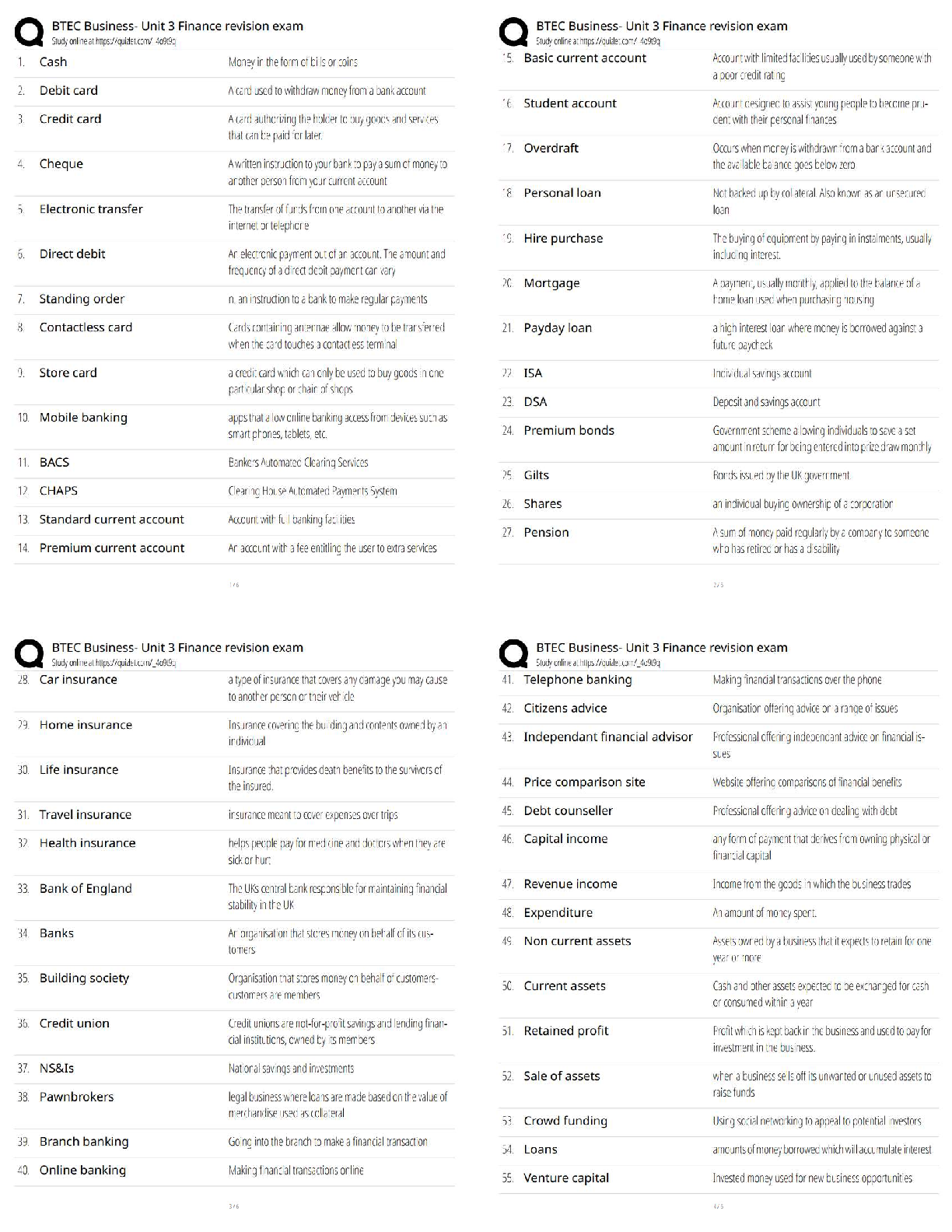
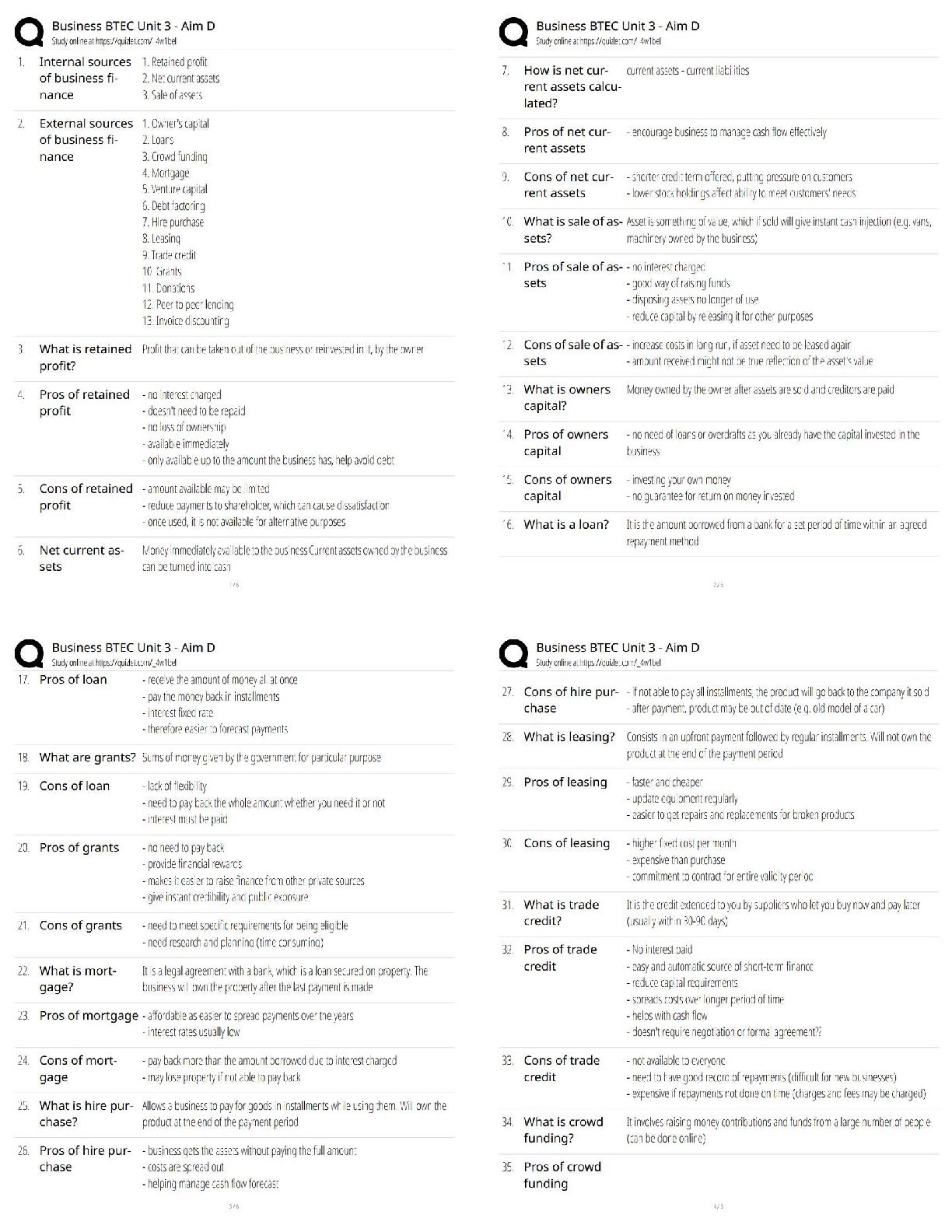
What is the purpose of an IRB? Correct Answer To protect the rights and welfare of human subjects of research When must the investigator obtain IRB approval of the study and the consent form? Corre ... ct Answer Before enrolling any patients in the study The IRB must inform the investigator the study has been approved by: Correct Answer Written notification saying it has been approved For initial approval of proposed research, the investigator must submit to the IRB: Correct Answer The full protocol and the informed consent Any proposed advertising for the study: Correct Answer Must be submitted to the IRB and approved before it can be used Any amendment that ____________ must be approved by the IRB prior to implementation. a. Increases the risk to subjects b. Decreases the number of subjects c. Changes the protocol in anyway d. All of the above e. None of the above Correct Answer A. Increases the risk to subjects Which of the following are necessary to waive consent? a. Subject is unable to give consent b. No time or unable to contact next of kin c. Life-threatening condition d. No other treatment available e. None of the above f. All of the above Correct Answer f. All of the above The investigator's signature must be on the consent form. True or false? Correct Answer False The process by which a subject voluntarily confirms his or her willingness to participate in a clinical trial is known as: Correct Answer Informed consent Informed consent is documented by: Correct Answer A written, signed, and dated informed consent form Which signatures are required by regulation to be on the consent form? a. The investigator b. The subject [Show More]
Last updated: 3 years ago
Preview 1 out of 7 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$6.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Dec 14, 2022
Number of pages
7
Written in
All
Additional information
This document has been written for:
Uploaded
Dec 14, 2022
Downloads
0
Views
111