SCIENCE 101 > QUESTIONS & ANSWERS > ACT Science ALL SOLUTION GUARANTEED GRADE A+ SPRING FALL -2023/24 EDITION GUARANTEED GRADE A+ (All)
ACT Science ALL SOLUTION GUARANTEED GRADE A+ SPRING FALL -2023/24 EDITION GUARANTEED GRADE A+
Document Content and Description Below
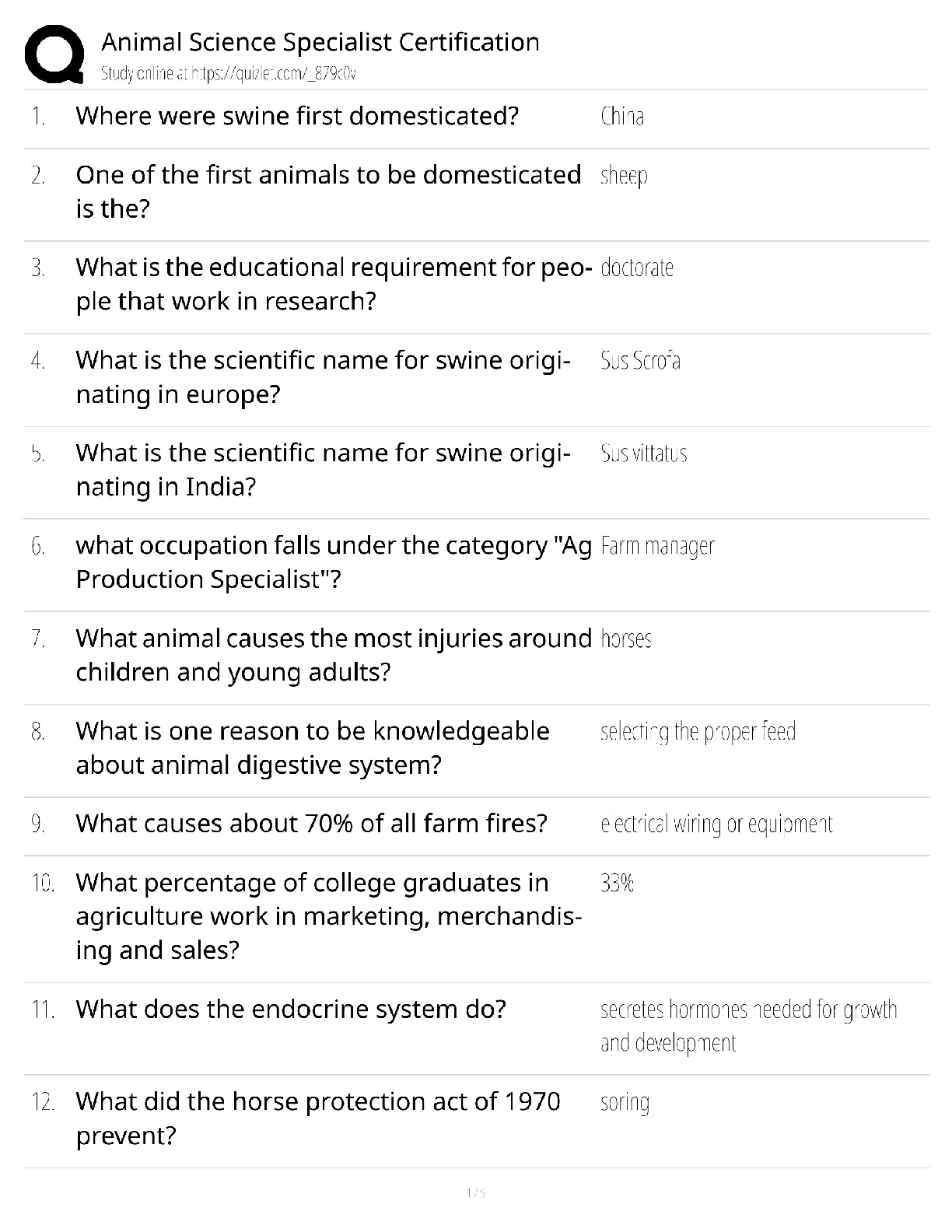
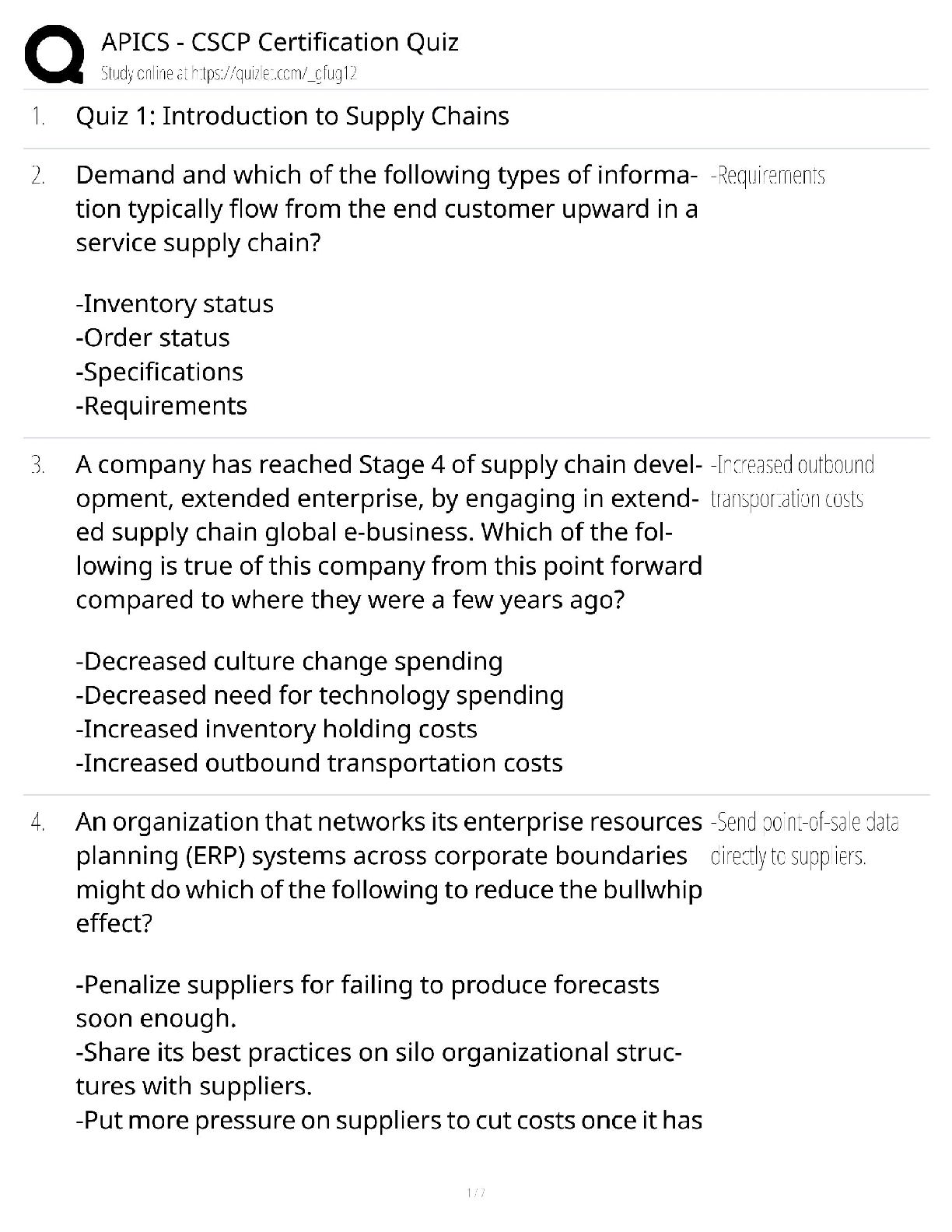
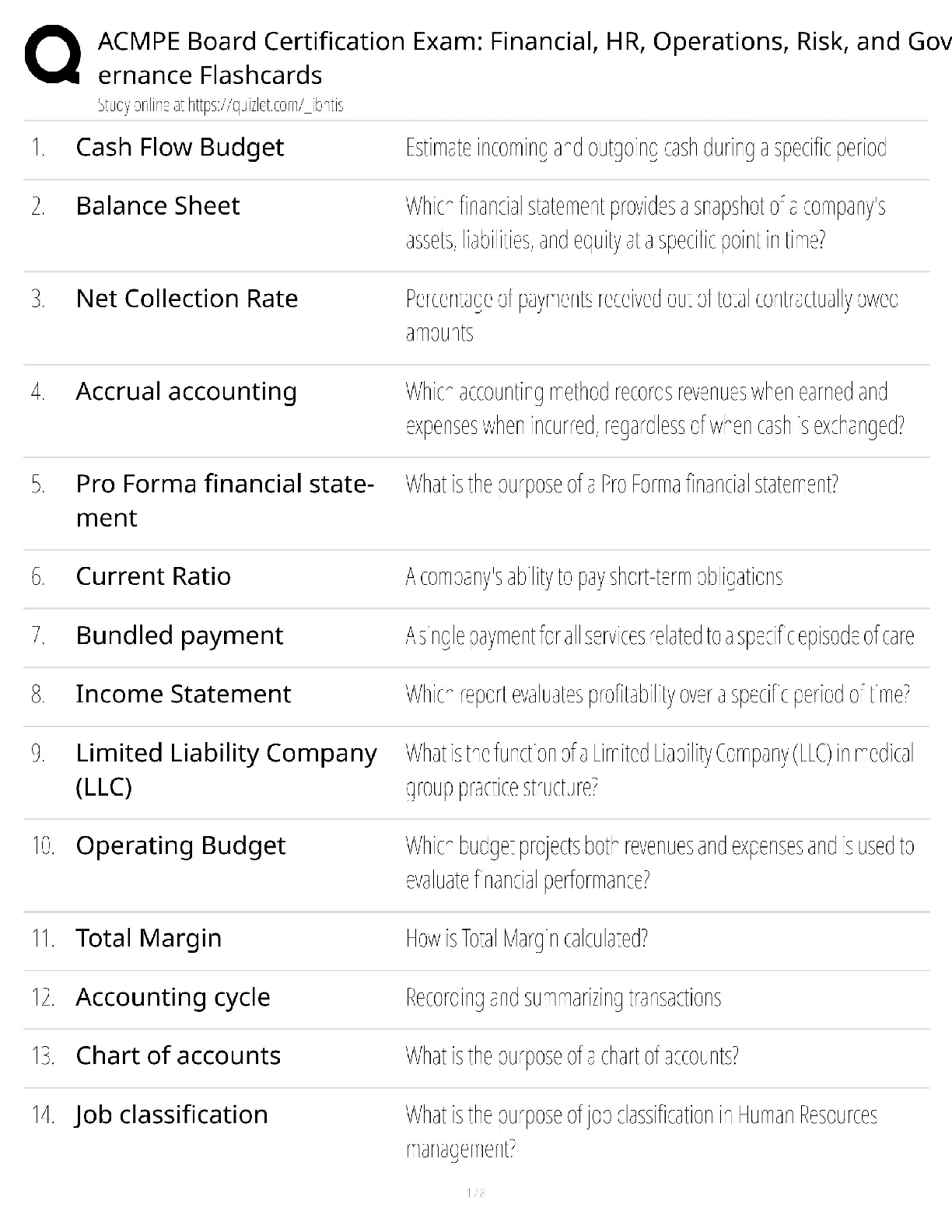
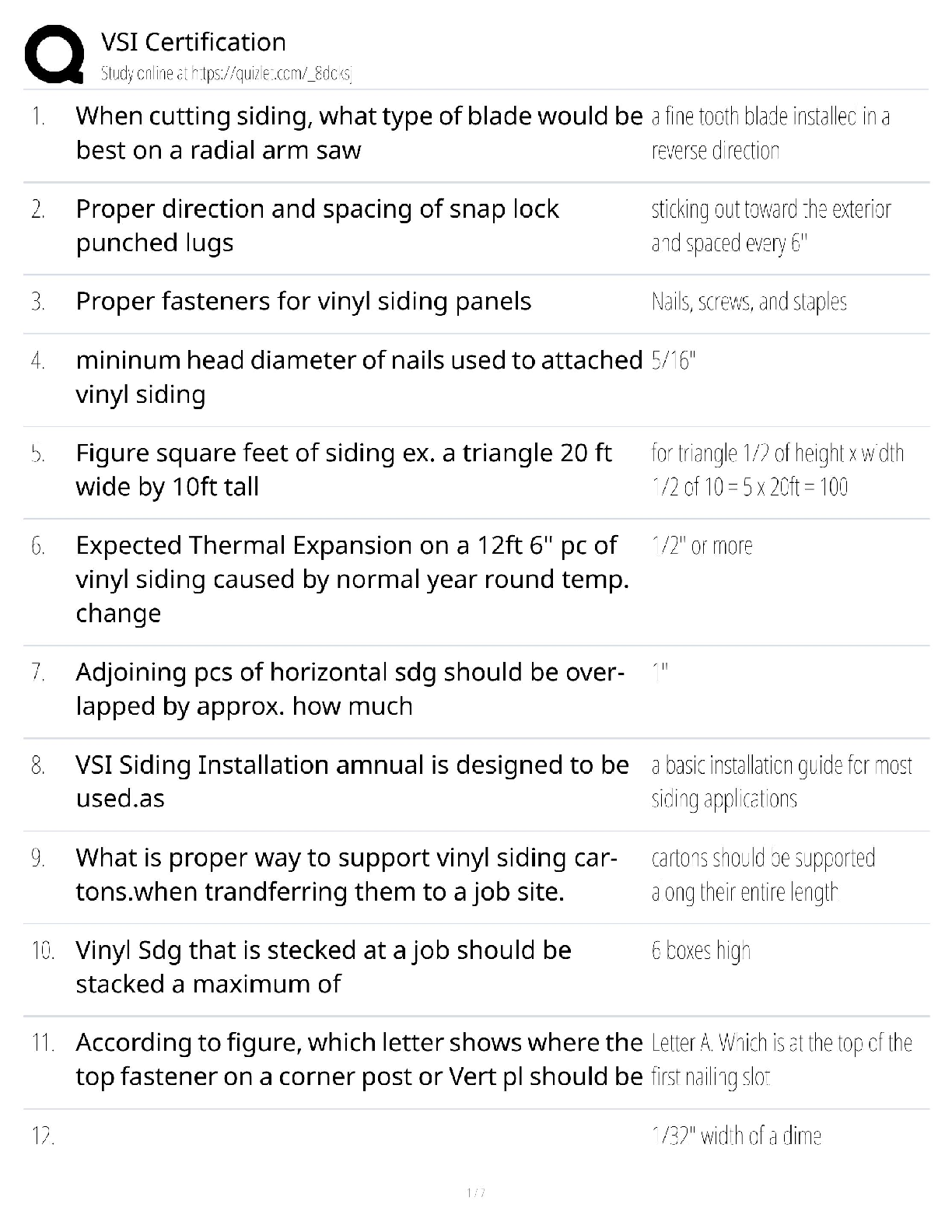
Based on the reading passage above and the information in the graph, it can be inferred that _____. number of protons = atomic number To remain stable, isotopes require _____. more neutrons as the ... number of protons increases Which statement is true when comparing an isotope with 17 protons to an isotope with 73 protons? The stable isotope of an atom with 73 protons requires a significantly higher percentage of neutrons than an atom with 17 protons for it to be stable. Radioactive isotopes always have _____. fewer neutrons than the stable isotope Why does a gold isotope, with an atomic number of 79, require more neutrons to be stable than a sodium isotope, with an atomic number of 11? More protons require more neutrons to keep the isotope stable. What is the relationship between volume and pressure in the graph? as pressure increases, volume decreases If the volume of the gas is 1 L, what will the pressure be? 6 atm At each data point on the graph, the value of V × P _____. is the same at all data points along the line How is the diagram below related to the graph? It shows the same relationship between pressure and volume. The graph below shows the relationship between volume (L) and pressure of a gas (atm). Using the information in the graph, what can be said about the nature of gases? What is the approximate elevation of point B? 85 ft Which statement is true about the points on the diagram? ? Which of the following terms most correctly identifies this topographical feature? Volcano Which statement is true about the topographical feature? it is steeper on one side than the other What elevation increment do the lines represent? 25 ft Bromothymol blue was added to a water sample from each location. Which statement describes the most likely results? The water sample from Location A turned yellow, while the water sample from Location B turned green. Which factor most directly affects the pH of the lake water at Sample Location B? presence of both animal and plant species Which biological process causes the pH of the water surrounding an organism to decrease? cellular respiration Which biological process causes the pH of the water surrounding an organism to increase? photosynthesis The scientist returned to Sample Location A at night and conducted the investigation again. This time, when bromothymol blue was added to the water sample, it had a more greenish-yellow appearance. What most likely accounts for this difference in results? At night, the water temperature drops, causing the amount of dissolved oxygen in the lake to rise. The scientist has a water sample from an unknown location in the lake. When bromothymol blue is added, the sample becomes blue. From where did the sample most likely come? neither Sample Location A nor Sample Location B The scientist tested another water sample at Sample Location C. When bromothymol blue was added to this sample, it immediately became yellow. What conclusion can be drawn from these results? [Show More]
Last updated: 2 years ago
Preview 1 out of 3 pages
Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$11.50
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Jan 12, 2023
Number of pages
3
Written in
All
Additional information
This document has been written for:
Uploaded
Jan 12, 2023
Downloads
0
Views
81