Exam >SCH4U UNIT 3 TEST: CHEMICAL EQUILIBRIUM ONTARIO VIRTUAL SCHOOL
Document Content and Description Below
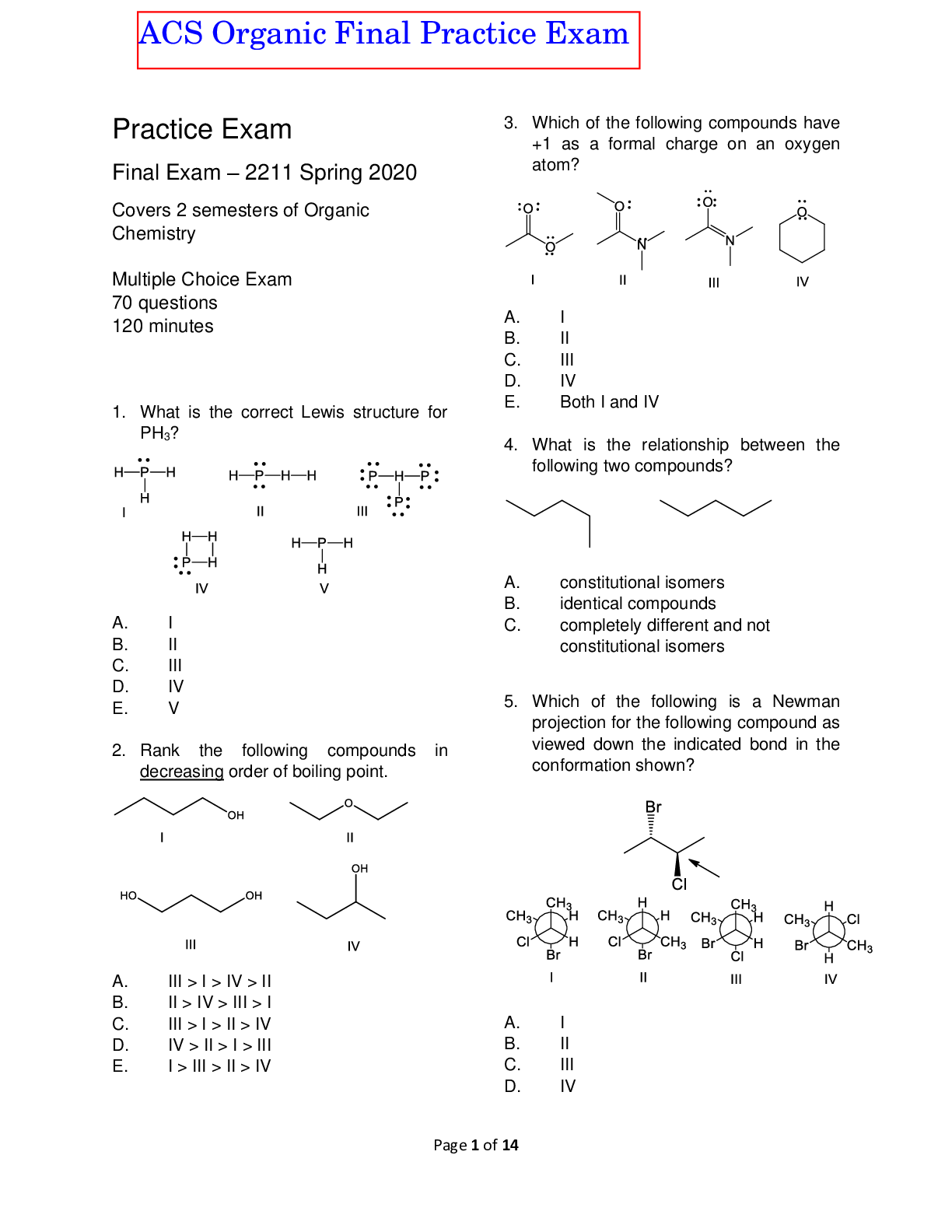
Part A: Knowledge/Understanding (16) 1. The graph to the right shows the progress of the reaction below at SATP. H2O(g) + CO(g) « H2(g) + CO2(g) The Keq of this reaction is: a. > 1 b. = 1 c.... < 1 d. not determinable from this graph 2. For the reaction 2NO(g) « N2O4(g), a decrease in pressure would result in: a. An increase in [N2O4] b. An increase in Keq c. An increase in Q d. An increase in [NO] 3. A reaction that is essentially irreversible would have: a. Keq >>1 b. 0 < Keq <<1 c. Keq << 0 d. Keq = 1 4. What is the correct Keq equation for the chemical equation below? Cu(S) + 2Ag+(aq) « Cu2+(aq) + 2Ag(s) Keq = 2 X 1015 at 25oC a. ["#!"][%&] ['("][)*] b. ["#!"][%&]! ['("]![)*] c. ["#!"] ['("]! d. ["#!"] ['("] 5. Which of the following statements is TRUE about this equilibrium? Cu(S) + 2Ag+(aq) « Cu2+(aq) + 2Ag(s) Keq = 2 X 1015 at 25oC a. If a piece of copper is placed in a solution of excess Ag2+ ions, it’s mass will eventually decrease to nearly 0. b. The reactants are favored in this reaction. c. Ag2+ will be in higher concentration than any other components. d. Silver must be higher on the activity series than copper. 6. Which change would increase the concentration of Pb2+ in the following equilibrium? PbCrO4(s) + heat « Pb2+(aq) + CrO42-(aq) a. increased temperature b. decreased temperature c. decreased pressure d. increased pressure 7. What is the molar solubility of Pb2+ in the following equilibrium (to 2 significant digits)? ___________________ PbCrO4(s) + heat « Pb2+(aq) + CrO42-(aq) Ksp = 2.0 x 10-16 at 25oC 8. In the following reaction, what are the two Bronsted-Lowry bases? ??#(%&) + ???(%&) ↔ ??()(%&) + ??(*%&) a. NH3 and HCl b. NH3 and NH4+ c. NH3 and Cld. HCl and NH4+ CH4U UNIT 3 TEST: CHEMICAL EQUILIBRIUM ONTARIO VIRTUAL SCHOOL 9. Which of the following is an amphoteric species? a. H2S(aq) b. H2SO4(aq) c. SO42-(aq) d. HSO4-(aq) 10. If 900 mL of water were added to a 100-mL solution of NaOH, the pH would: a. increase by 10 b. decrease by 10 c. increase by 1 d. decrease by 1 e. it depends on the original concentration of NaOH 11. Which of the following pairs of compounds could be used to make a buffer solution? a. HNO3 and NaNO3 b. NH3 and NH4Cl c. NaOH and NaHC2O4 d. H2(PO4)- and PO43- 12. Nitrogen dioxide is the cause of photochemical smog, the brownish haze produced by car exhaust that hangs over many cities. It exists in an equilibrium with dinitrogen tetroxide, which is colourless, as shown below. N2O4(g) + energy « 2NO2(g) colourless reddish brown Based on this equilibrium, smog would be worse: a. at higher air pressures b. when there’s less traffic c. in the summer time d. in the winter time 13. In a study of the equilibrium described above, an environmental chemist finds that, at a certain temperature, a closed flask contains 2.00 mmol/L of NO2(g) and 5.00 mmol/L of N2O4(g). The numerical value for the equilibrium constant at this temperature is: a. 0.800 b. 0.400 c. 2.50 d. 1.25 14. Ice cubes are melting in a covered bottle of water at room temperature. What is true about this system? a. It represents an equilibrium system, because the room is a constant temperature b. It does not represent an equilibrium system, because the temperature is not constant c. It represents an equilibrium system, because melting and freezing are happening at equal rates d. It does not represent an equilibrium system, because there isn’t an equal rate of melting and freezing 15. A reaction quotient is calculated to be ? = 3.0 × 10*(. The equilibrium constant for the same reaction is ? +& = 7.2 × 10*(. Which statement is correct? a. The system is at equilibrium. b. The concentrations of the products are greater than the concentrations of the reactants. c. The system will attain equilibrium by moving to the right. d. The system will attain equilibrium by moving to the left. 16. For the following equilibrium, what will happen if potassium chlorate is added to the system? a. The solution will become more pink. b. The solution will become more blue. c. The pH of the system will increase. d. There will be no change to the system. [Show More]
Last updated: 2 years ago
Preview 1 out of 7 pages
Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$4.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Jan 14, 2023
Number of pages
7
Written in
Additional information
This document has been written for:
Uploaded
Jan 14, 2023
Downloads
0
Views
60






.png)


.png)




