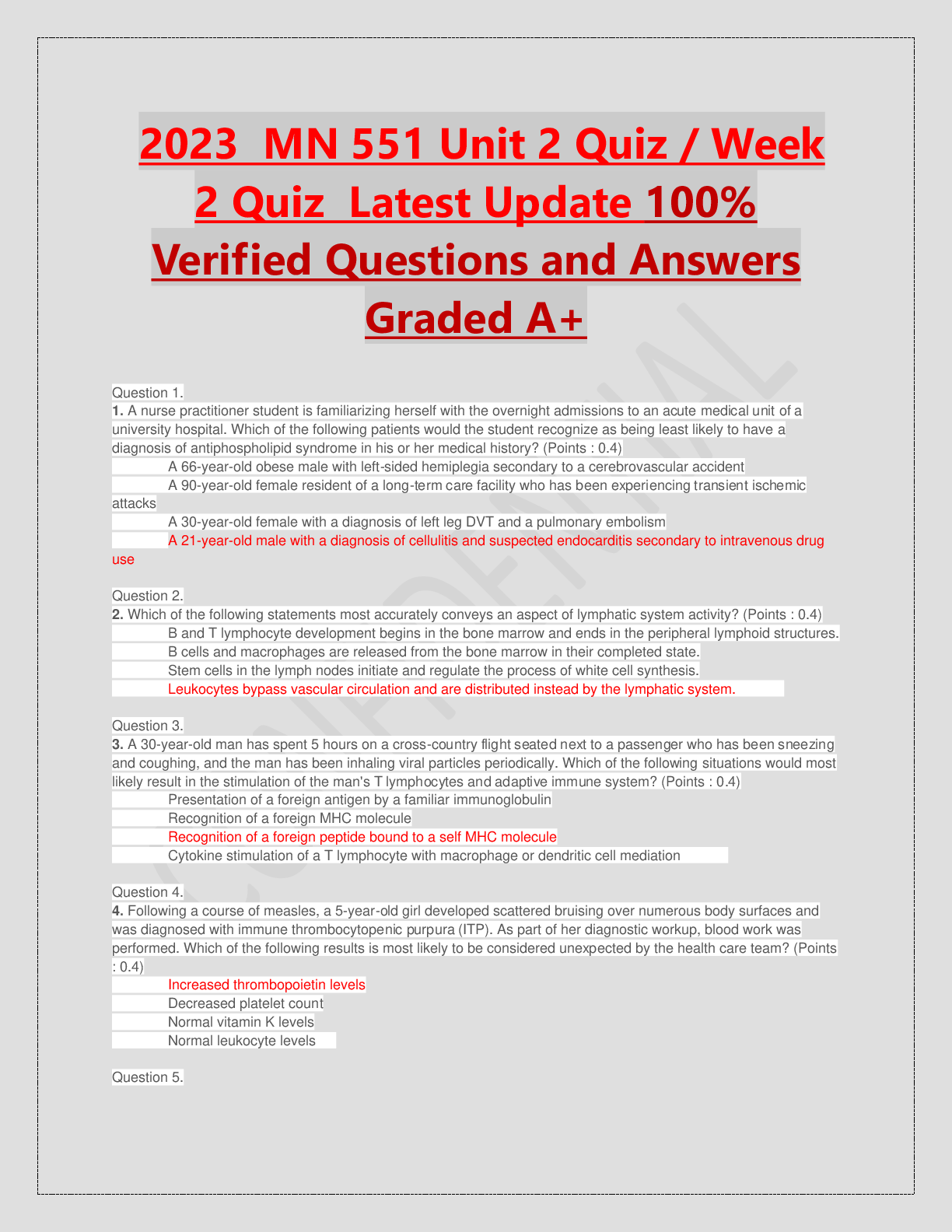
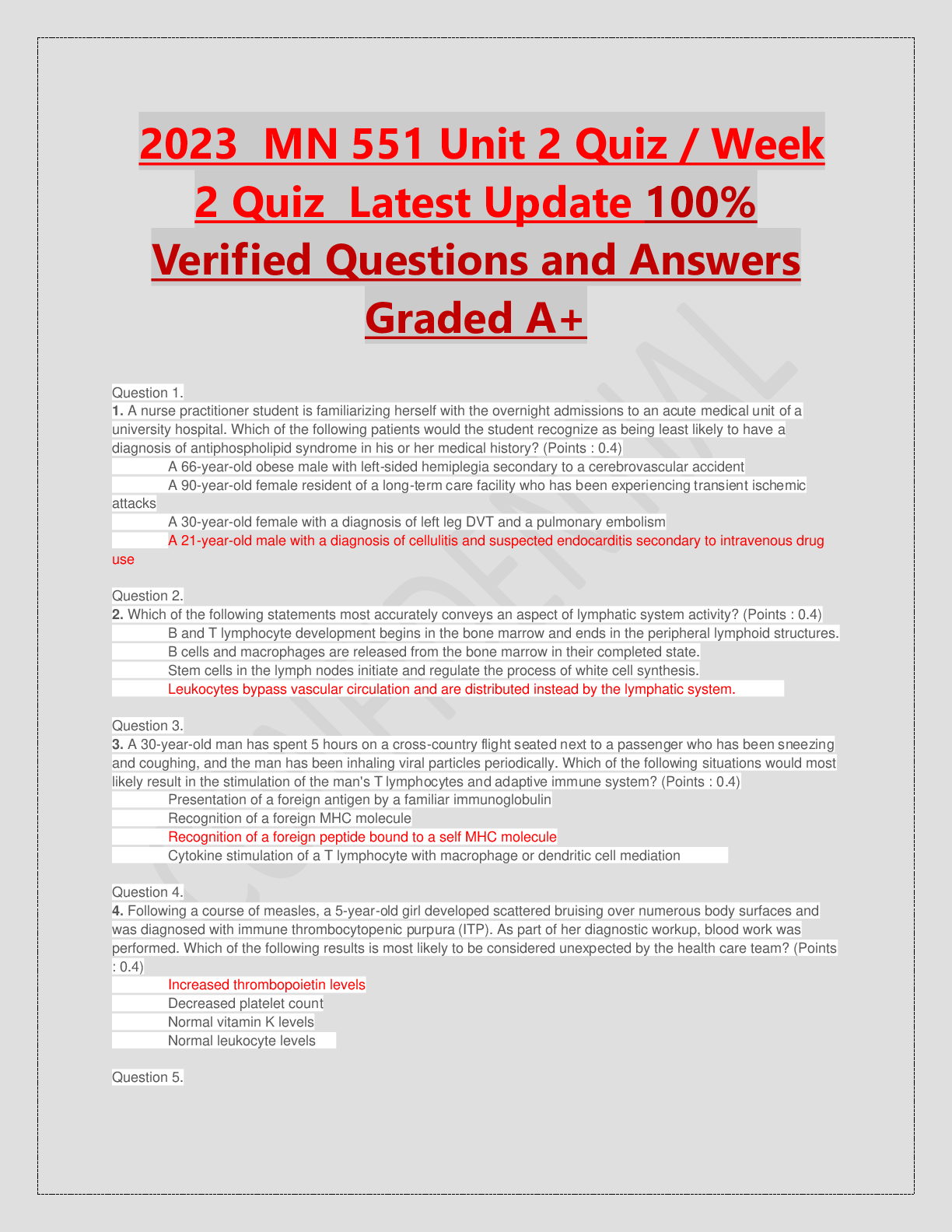
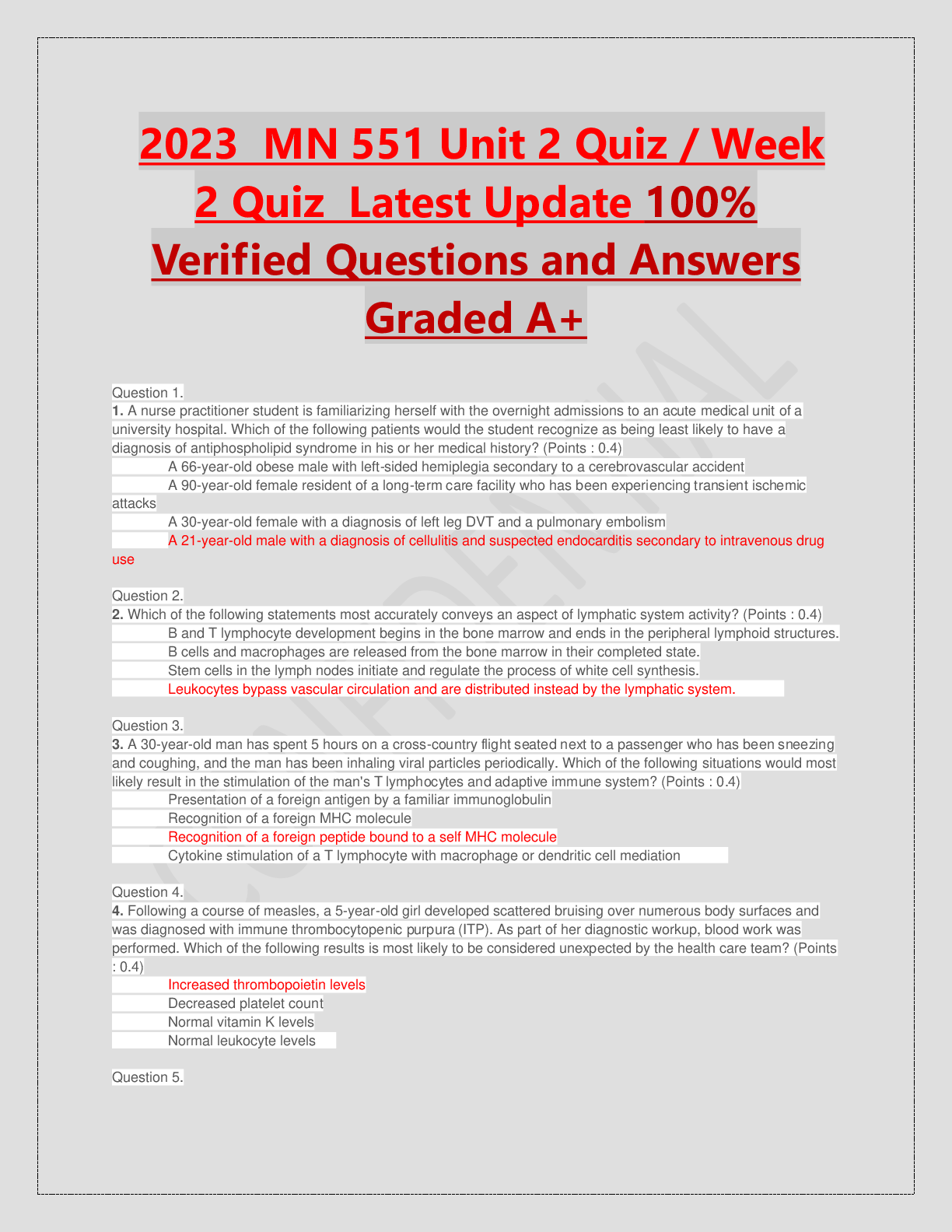
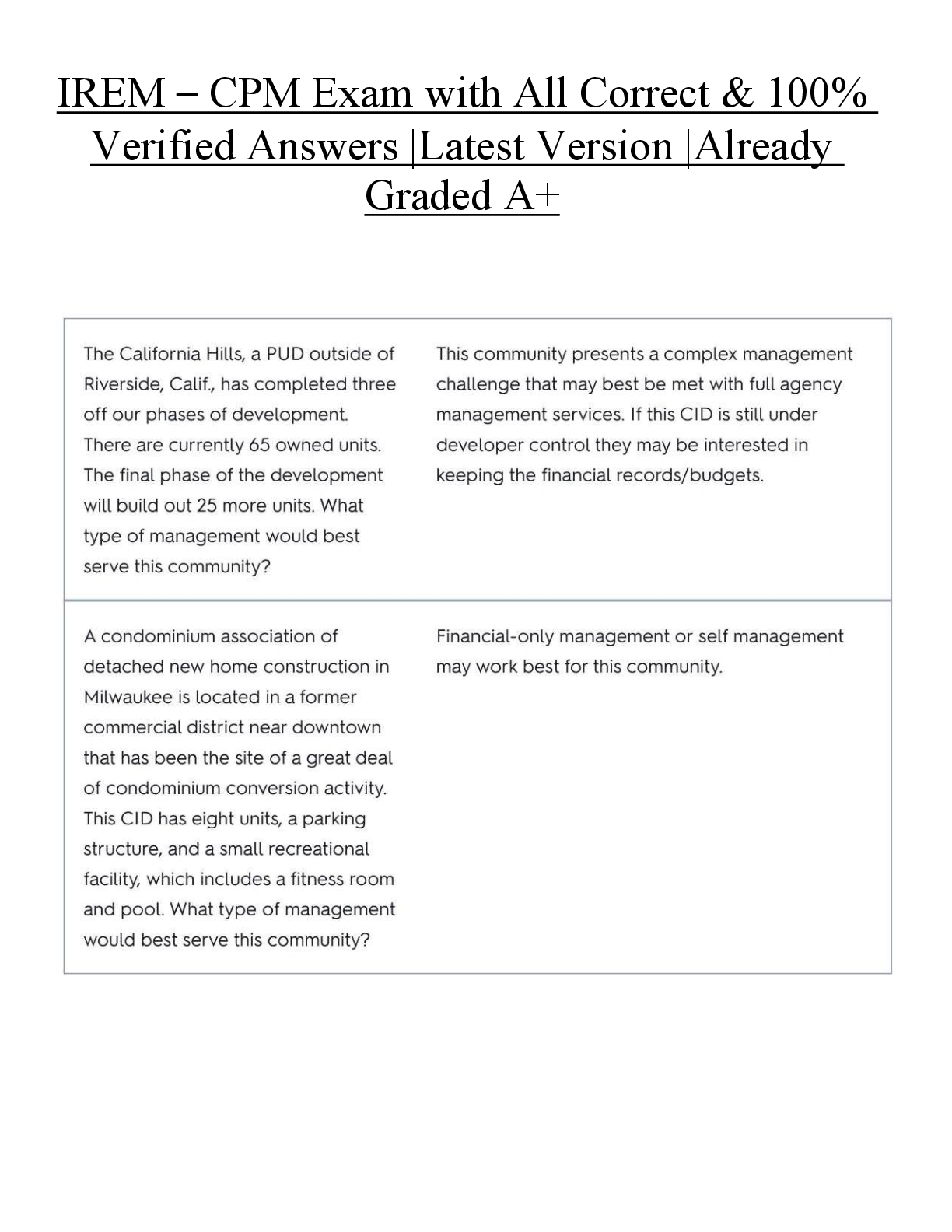
ACRP CCRA/CCRC Certification Exam prep written March 2023
Adverse Event (AE)/Adverse Experience - answerAny untoward medical occurrence in a patient or clinical investigation subject administered a pharmaceutical p
...
ACRP CCRA/CCRC Certification Exam prep written March 2023
Adverse Event (AE)/Adverse Experience - answerAny untoward medical occurrence in a patient or clinical investigation subject administered a pharmaceutical product and which does not necessarily have to have a causal relationship with this treatment.
Adverse Drug Reaction (ADR) - answerAll noxious and unintended response to a medicinal product related to any dose.
Unexpected Adverse Drug Reaction - answerAn adverse reaction, the nature or severity of which is not consistent with the applicable product information (e.g., Investigator's Brochure for an unapproved investigational medicinal product). (See Section III.c)
Serious Adverse Event (Experience) or Reaction - answerIs any untoward medical occurrence that at any dose:
Results in death,
Is life-threatening
"life-threatening" in the definition of "serious" - answerIn the definition of "serious" refers to an event in which the patient was at risk of death at the time of the event; it does not refer to an event which hypothetically might have caused death if it were more severe.
Requires inpatient hospitalization or prolongation of existing hospitalization,
Results in persistent or significant disability/incapacity,
or
Is a congenital anomaly/birth defect.
Marketed Medicinal Products - answerA response to a drug which is noxious and unintended and which occurs at doses normally used in man for prophylaxis, diagnosis, or therapy of disease or for modification of physiological function.
"Unexpected" Adverse Event/Reaction - answerThe nature or severity of which is not consistent with information in the relevant source document(s). Until source documents are amended, expedited reporting is required for additional occurrences of the reaction.
"Expected" Adverse Event/Reaction - answerFor a medicinal product not yet approved for marketing in a country, a company's Investigator's Brochure will serve as the source document in that country
[Show More]