Combined Science: Synergy > QUESTION PAPER (QP) > Pearson Edexcel Level 1/Level 2 GCSE (9-1) Time 1 hour 10 minutes Paper reference Combined Science P (All)
Pearson Edexcel Level 1/Level 2 GCSE (9-1) Time 1 hour 10 minutes Paper reference Combined Science PAPER 2 Foundation Tier
Document Content and Description Below
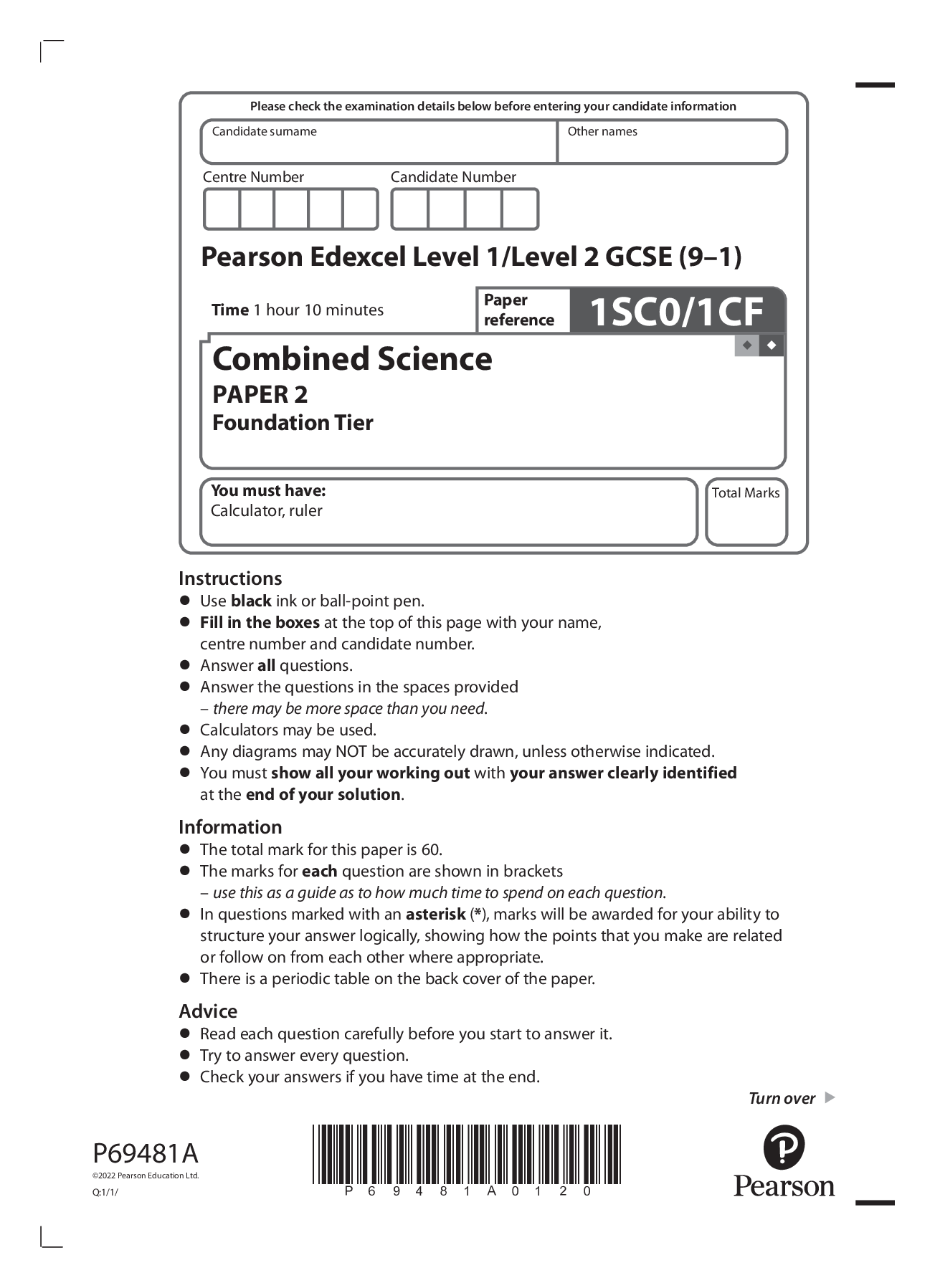
Instructions • Use black ink or ball-point pen. • Fill in the boxes at the top of this page with your name, centre number and candidate number. • Answer all questions. • Answer the quest... ions in the spaces provided – there may be more space than you need. • Calculators may be used. • Any diagrams may NOT be accurately drawn, unless otherwise indicated. • You must show all your working out with your answer clearly identified at the end of your solution. Information • The total mark for this paper is 60. • The marks for each question are shown in brackets – use this as a guide as to how much time to spend on each question. • In questions marked with an asterisk (*), marks will be awarded for your ability to structure your answer logically, showing how the points that you make are related or follow on from each other where appropriate. • There is a periodic table on the back cover of the paper. Advice • Read each question carefully before you start to answer it. • Try to answer every question. • Check your answers if you have time at the end. *P69481A0220* 2 Answer ALL questions. Write your answers in the spaces provided. Some questions must be answered with a cross in a box . If you change your mind about an answer, put a line through the box and then mark your new answer with a cross . 1 Figure 1 shows a metal spoon and two test tubes being heated in a water bath. One test tube contains a piece of chocolate, the other some liquid egg white. metal spoon chocolate egg white heat Figure 1 After heating, the spoon, the chocolate and the egg white are allowed to cool to room temperature. Figure 2 shows the state of the three different substances before heating, when hot and after cooling. substance before heating when hot after cooling metal spoon solid solid solid chocolate solid liquid solid egg white liquid solid solid Figure 2 *P69481A0320* Turn over 3 (a) Describe the differences in the arrangement and movement of the particles in a solid and in a liquid. (2) difference in arrangement of particles ................................................................................................................................................................................................................................................................................... .................................................................................................................................................................................................................................................................................... .................................................................................................................................................................................................................................................................................... difference in movement of particles .................................................................................................................................................................................................................................................................................... .................................................................................................................................................................................................................................................................................... .................................................................................................................................................................................................................................................................................... (b) What name is given to the process when the chocolate changes from a solid to a liquid? (1) A condensing B evaporating C freezing D melting (c) Give a reason why the metal spoon has not changed state during the experiment. (1) .................................................................................................................................................................................................................................................................................... .................................................................................................................................................................................................................................................................................... (d) Explain how we know the change to the egg white is a chemical change rather than a physical change. (2) .................................................................................................................................................................................................................................................................................... .................................................................................................................................................................................................................................................................................... .................................................................................................................................................................................................................................................................................... .................................................................................................................................................................................................................................................................................... (Total for Question 1 = 6 marks) *P69481A0420* 4 2 Potable water is water that is suitable for drinking. (a) River water can be treated to make it potable. Chlorination, filtration and sedimentation are three of the processes involved in making the river water potable. (i) Which row of the table shows these three processes in the order in which they are carried out? (1) A B C D first second third chlorination sedimentation filtration chlorination filtration sedimentation sedimentation filtration chlorination sedimentation chlorination filtration (ii) State the reason why chlorine is added during the water treatment. (1) .................................................................................................................................................................................................................................................................................... .................................................................................................................................................................................................................................................................................... (iii) Describe how sedimentation is carried out. (2) .................................................................................................................................................................................................................................................................................... .................................................................................................................................................................................................................................................................................... .................................................................................................................................................................................................................................................................................... .................................................................................................................................................................................................................................................................................... *P69481A0520* Turn over 5 (iv) Figure 3 shows the results of an analysis of a sample of potable water. ion concentration in mg dm−3 chloride 60.70 fluoride 0.24 nitrate 24.90 sulfate 71.40 copper 0.05 magnesium 9.10 Figure 3 Using this information, explain why this sample of potable water is not the same as pure water. (2) .................................................................................................................................................................................................................................................................................... .................................................................................................................................................................................................................................................................................... .................................................................................................................................................................................................................................................................................... .................................................................................................................................................................................................................................................................................... *P69481A0620* 6 (b) A student wanted to distil a sample of potable water. Figure 4 shows apparatus the student used. heat potable water X Figure 4 (i) Name the piece of equipment labelled X in Figure 4. (1) .................................................................................................................................................................................................................................................................................... (ii) The student made an error when setting up the equipment in Figure 4. This error meant no water could be collected in the test tube. Explain what the student needs to do so water can be collected. (2) .................................................................................................................................................................................................................................................................................... .................................................................................................................................................................................................................................................................................... .................................................................................................................................................................................................................................................................................... .................................................................................................................................................................................................................................................................................... [Show More]
Last updated: 2 years ago
Preview 1 out of 20 pages
Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$7.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Apr 03, 2023
Number of pages
20
Written in
Additional information
This document has been written for:
Uploaded
Apr 03, 2023
Downloads
0
Views
141









.png)