Chemistry > QUESTIONS & ANSWERS > CHEM1010H – Report 5: Esterification _ Trent University. Chem1010-Lab5-Report Template Q&A (All)
CHEM1010H – Report 5: Esterification _ Trent University. Chem1010-Lab5-Report Template Q&A
Document Content and Description Below
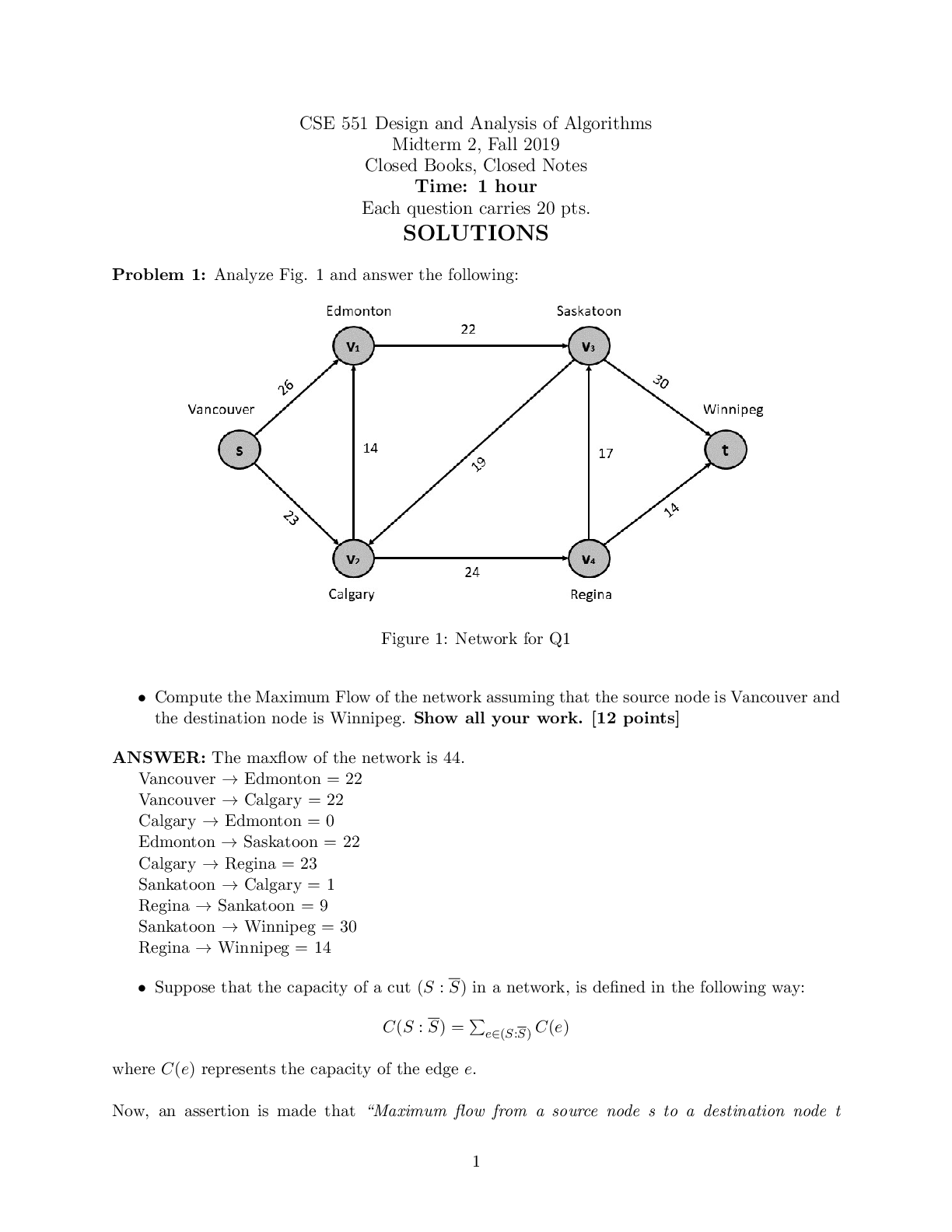
CHEM1010H – Report 5: Esterification 1. Provide your raw data for the synthesis of ASA ANSWER Mass of salicylic acid and weighing paper: 2.3507g Mass of weighing paper after transfer: 0.2239g M... ass of salicylic acid: 2.1268g Mass of empty 100mL beaker: 49.8921g Mass of dried product in 100mL beaker: 51.6700g Mass of dried product: 1.7779g Melting point range of your product: 105.4C – 146.3C 2. Show that ethanoic anhydride is in excess, for your preparation of ASA. You will need to briefly, but completely, explain the result of your calculation See note ANSWER: Density of ethanoic anhydride: 1.092 g/mL Ethanoic anhydride used: 2.5 mL M = D x V = 1.092 g/mL x 2.5mL = 2.73g Mole Ratio = 1:1:1 of salicylic acid and ethanoic anhydride Moles of salicylic acid = mass/molar mass = 1.7779g/138.118g/mol = 0.01287mol Moles of Ethanoic anhydride = mass/molar mass [Show More]
Last updated: 2 years ago
Preview 1 out of 4 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$9.50
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Apr 09, 2023
Number of pages
4
Written in
Additional information
This document has been written for:
Uploaded
Apr 09, 2023
Downloads
0
Views
70