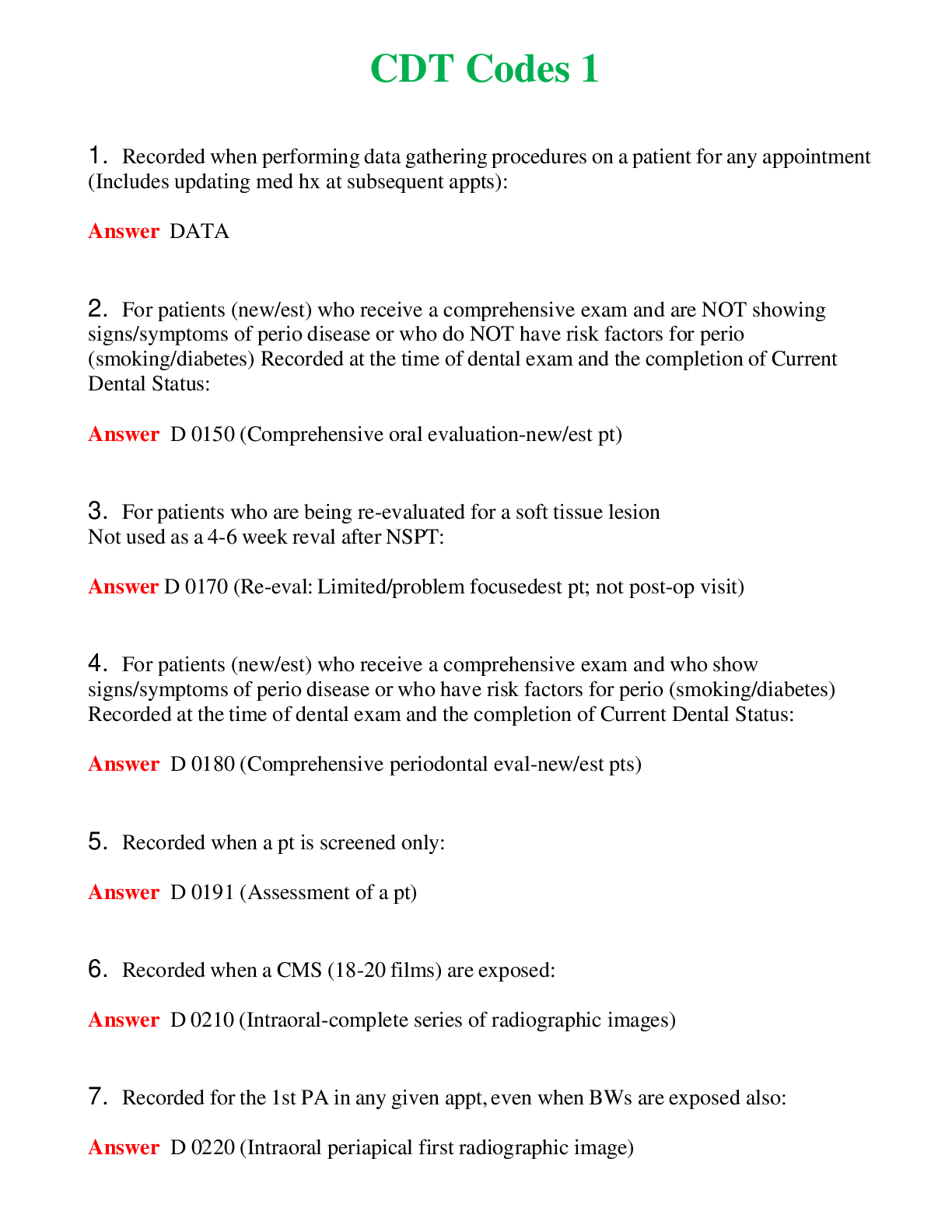
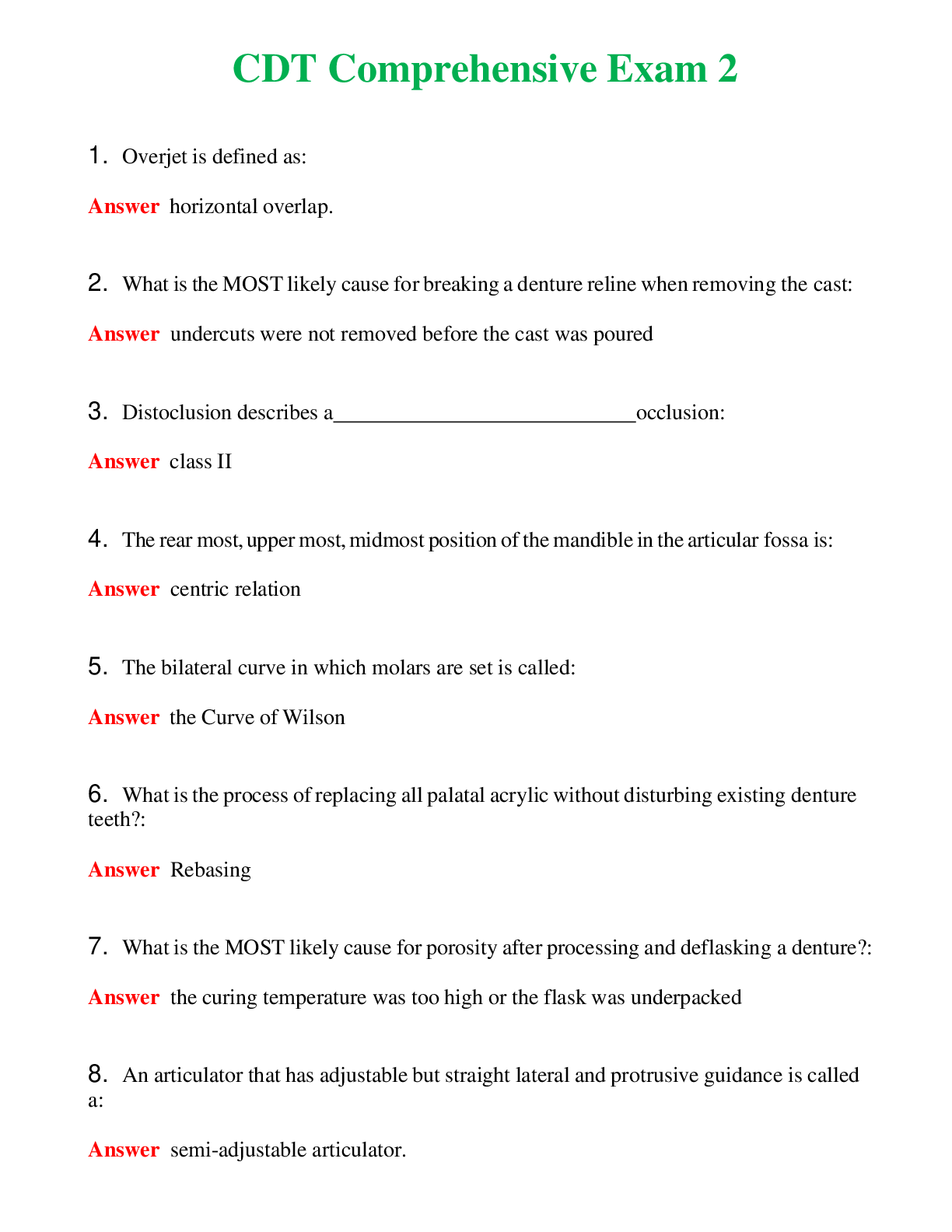
CHEM 120 Unit 8 Final Exam (Version 2) complete solutions, Latest 2019/20.
Document Content and Description Below
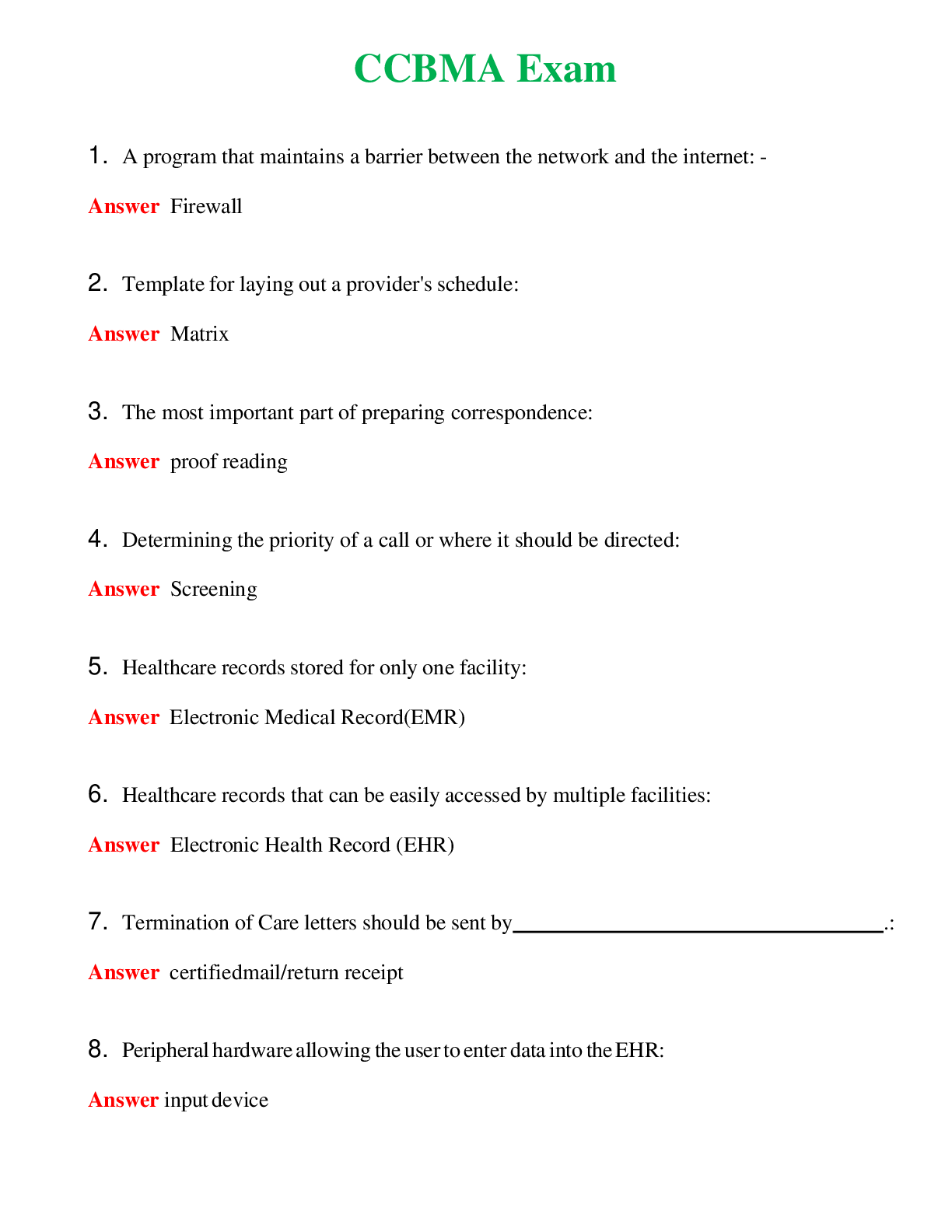
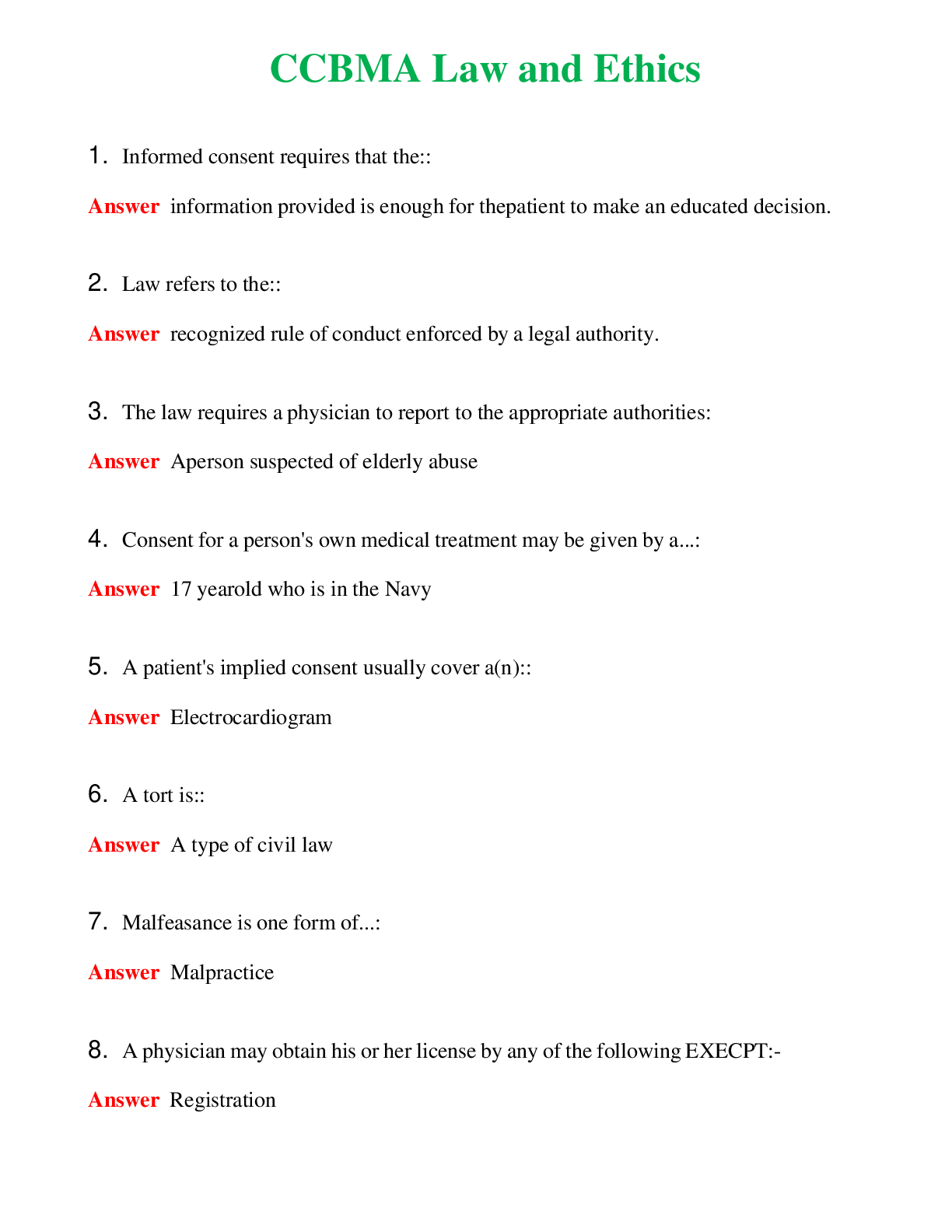
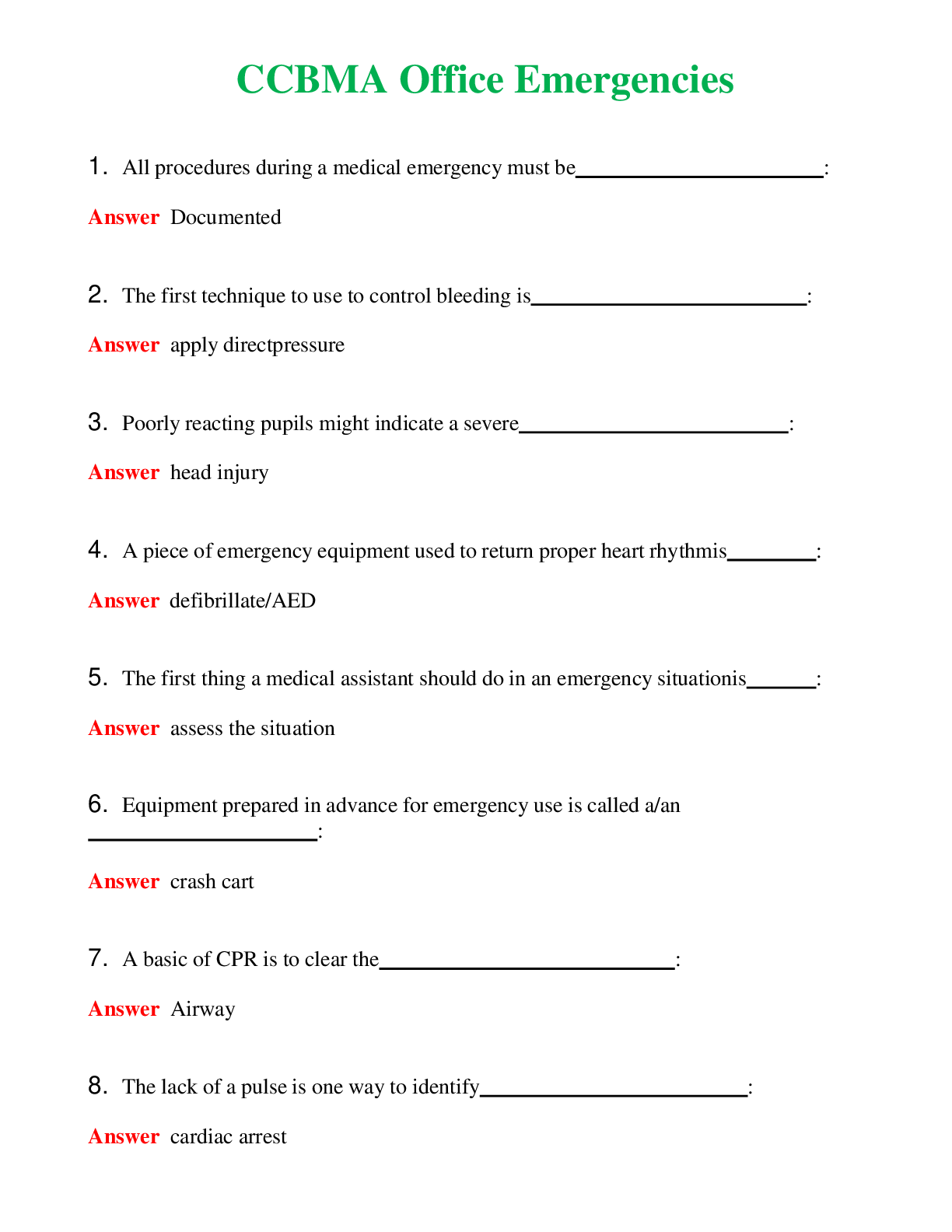
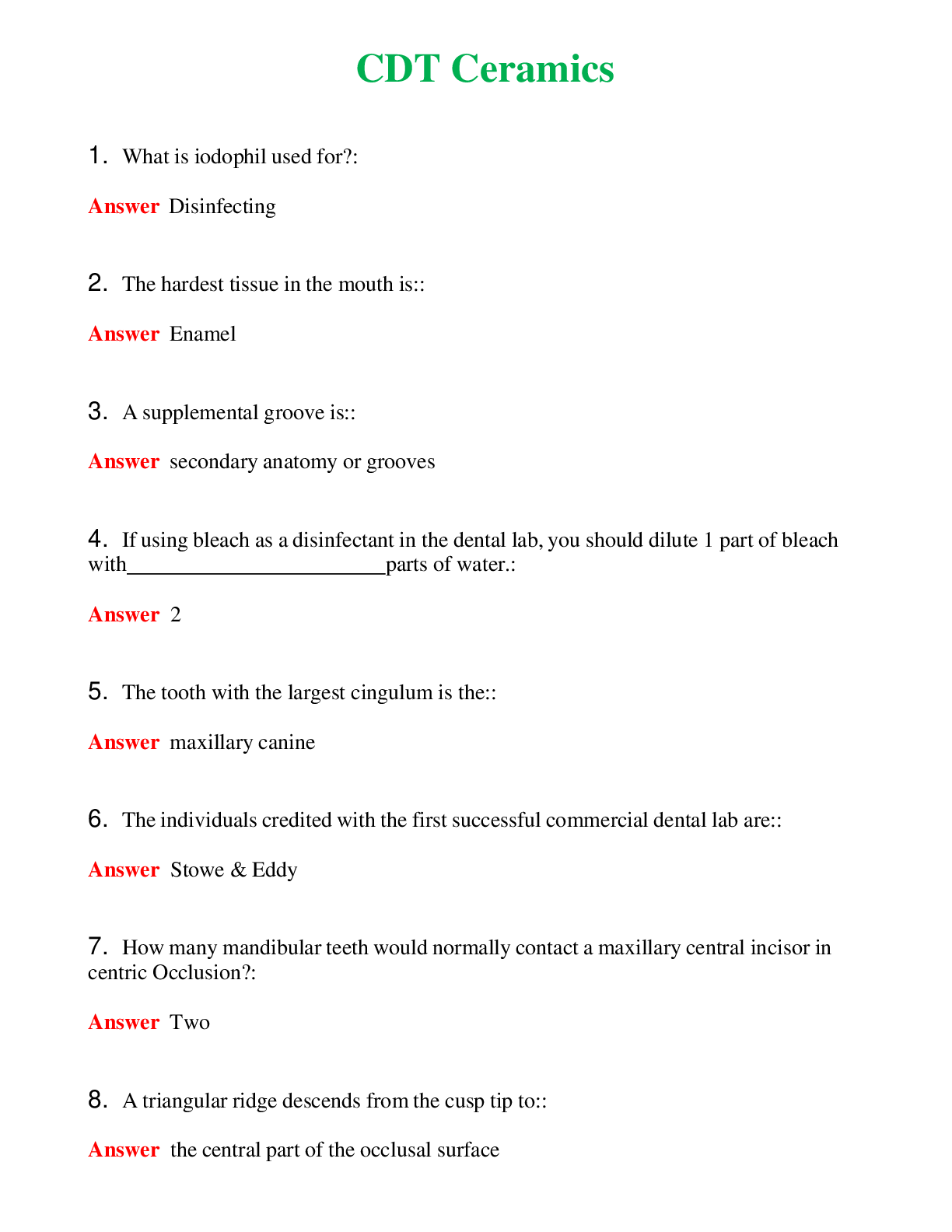
CHEM 120 Unit 8 Final Exam (Version 2) Chamberlain College of Nursing 1. Question (TCO 4) The shape of a carbon dioxide molecule is _____. 2. Question (TCO 4) Which of the following substances does ... not have polar covalent bonds? 3. Question (TCO 8) According to the Bronsted-Lowry definition, which chemical in the following reaction is the acid? 4. Question (TCO 8) According to the Bronsted-Lowry definition, which chemical in the following reaction is the base? 5. Question (TCO 12) Which nucleic acid carries the amino acid that is added onto the growing amino acid chain during translation? 6. Question (TCO 12) Which of the following best describes the action of enzymes in living systems? A specific enzyme generally catalyzes 7. Question (TCO 12) Which of the following is not an unsaturated fatty acid? 8. Question (TCO 12) The secondary structure of a protein is determined by 9. Question (TCO 12) Which of the following is not a monosaccharide? 10. Question (TCO 12) Cell nutrients and waste must pass through the 11. Question (TCO 12) Which of the following is a monosaccharide? 12. Question (TCO 2) Match the atom with the appropriate description. 13. Question (TCO 10) Match the organic compound with the functional group it has. 14. Question (TCO 9) Match the organic compound with its name. 15. Question (TCO 8) 75.0 mL of 1.45 M NaOH is neutralized by 93.7 mL of an HCl solution. What is the molarity of the HCl solution? (Show your work.) 16. Question (TCO 6) A sample of helium gas occupies 945 mL at 605 mmHg. For a gas sample at a constant temperature, determine the volume of helium at 745 mmHg. Show your work. 17. Question (TCO 6) A gas at a temperature of 195 degrees C occupies a volume of 165 mL. Assuming constant pressure, determine the volume at 45 degrees C. Show your work. 18. Question (TCO 6) Calculate the volume, in liters, of a 4.75 mol H2S(g) at 90 degrees C and 1.75 atm. Show your work. 19. Question (TCO 1) How many grams are in 1.7 pounds? Show your work. 20. Question (TCO 3) For the following molecule, give the cation name and symbol (1pt) , anion name and symbol, type(s) of bond and name of the molecule AlPO4 21. Question (TCO 12) If one strand of a DNA double helix has the sequence GCCACTGATA, what is the sequence of the other DNA strand? 22. Question (TCO 7) (a) Given that the molar mass of H3PO4 is 97.994 grams, determine the number of grams of H3PO4 needed to prepare 1.50 L of a 0.25 M H3PO4 solution. Show your work. (b) What volume, in liters, of a 0.25 M H3PO4 solution can be prepared by diluting 50 mL of a 1.75 M H3PO4 solution? Show your work. 23. Question (TCO 11) Hafnium (Hf), with a mass number of 174 and an atomic number of 72, decays by emission of an alpha particle. Identify the product of the nuclear reaction by providing its atomic symbol, mass number, and atomic number. 24. Question (TCO 5) Provide answers for (a)–(d) based the following unbalanced chemical equation. Al + Cl2-> AlCl3 25. Question (TCO 13) What is the mRNA sequence for the following segment of DNA? [Show More]
Last updated: 3 years ago
Preview 1 out of 5 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$11.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Mar 11, 2020
Number of pages
5
Written in
All
Additional information
This document has been written for:
Uploaded
Mar 11, 2020
Downloads
0
Views
200