AQA A Level Chemistry: Year 1- Alkenes
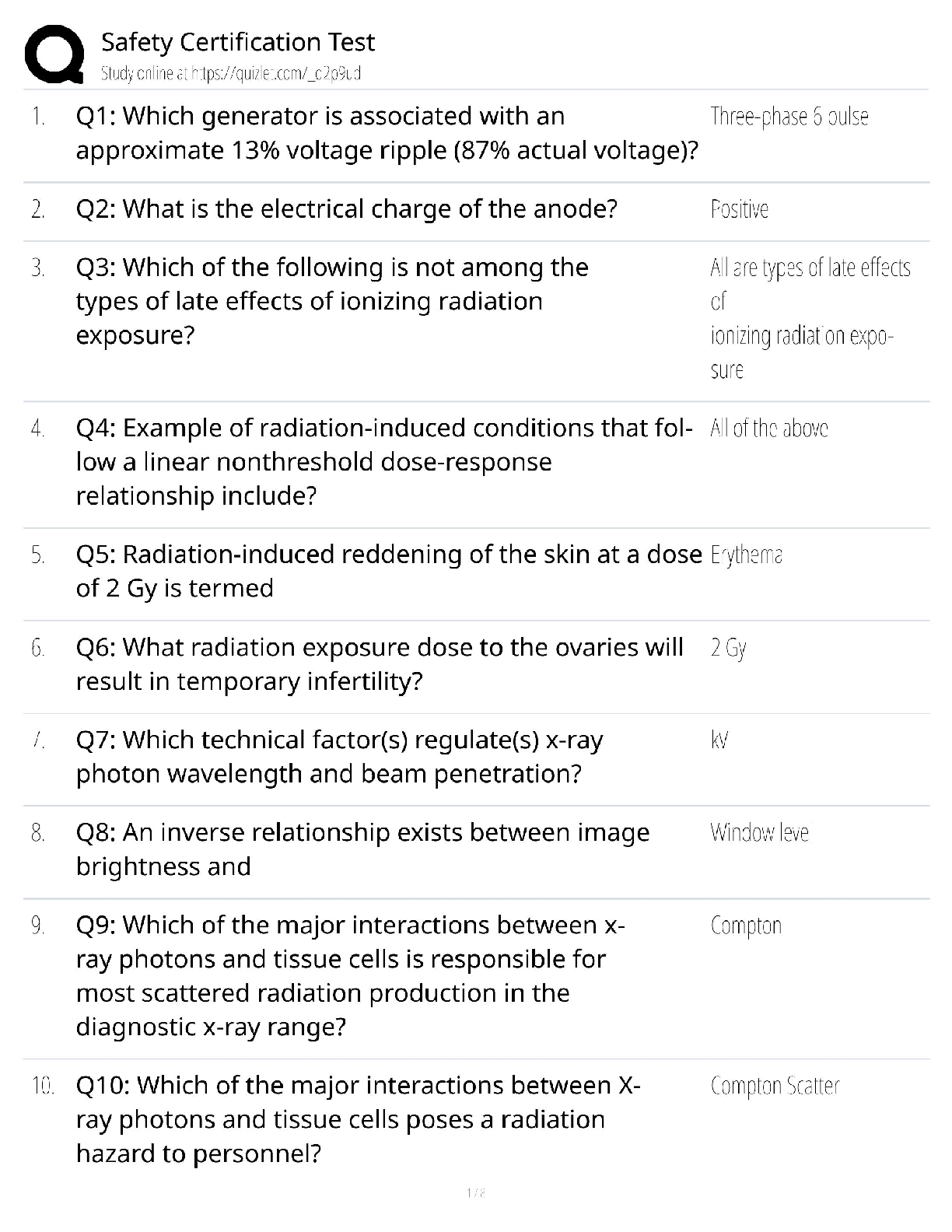
Already Passed
What is an alkene? ✔✔An unsaturated hydrocarbon
What is an unsaturated hydrocarbon? ✔✔A compound containing only carbon and hydrogen
atoms and containing at least
...
AQA A Level Chemistry: Year 1- Alkenes
Already Passed
What is an alkene? ✔✔An unsaturated hydrocarbon
What is an unsaturated hydrocarbon? ✔✔A compound containing only carbon and hydrogen
atoms and containing at least one carbon carbon double bond
What are van der Waals forces? ✔✔Relatively weak intermolecular forces caused by temporary
dipole dipole interaction
What is the general formula for an alkene? ✔✔CnH2n
How would you name an alkene given its displayed formula? ✔✔Identify the longest carbon
chain
Identify the position of the carbon carbon double bond
Identify any side chains
What are structural isomers? ✔✔Molecules with the same molecular formula, but with different
a structural formula
What are stereoisomers? ✔✔Molecules with the same molecular AND structural formula but a
different 3D arrangement of their atoms in space
What is E-Z isomerism? ✔✔The highest priority groups are on the same side of the double bond
Describe the C=C double bond ✔✔Double covalent bond
Planar bond
Area of high electron density
How do you assign the highest priority of groups in E-Z isomerism? ✔✔The highest atomic
number closest to the double bond has the highest priority
If two groups have e.g. Only carbon and hydrogen, then the one with the most carbon atoms is
assigned the highest priority
What are Z isomers? ✔✔Molecules where the highest priority groups are on the same side
What are E isomers? ✔✔Molecules where the highest priority groups are on opposite sides
What is addition polymerisation? ✔✔The reaction by which alkenes (or substituted alkenes)
react with other alkene (or substituted alkene) molecules to form polymers
What polymer would the monomer ethene form? ✔✔Poly(ethene)
What is a polymer? ✔✔A long chain of repeating monomer units joined together
Why are addition polymers unreactive? ✔✔They lack the reactive carbon-carbon double bond
Which is an area of high electron density
And so vulnerable to electrophilic attack
What is PVC? ✔✔Poly(chloroethene)
What is PVC used for? ✔✔PVC: Wellies, raincoats,
uPVC: Drainpipes, window/door frames
Electrical wire insulation
Describe PVC ✔✔A rigid plastic
What is the difference between uPVC and PVC? ✔✔A plasticiser molecule has been added to
PVC to make it more flexible for its use in clothing
What are the forces between polymer molecules? ✔✔Weak van der Waals' forces
Why do some polymers melt over a range of temperatures? ✔✔Consist of chains of different
lengths
varying strength of van der Waals'
When will a polymer melt? ✔✔When the (intermolecular) van der Waals forces are overcome
Why are the intermolecular forces between polymers often strong? ✔✔Chains are often long
Many electrons
Many van der Waals'
Explain why a tertiary carbocation forms the major product and a secondary/primary carbocation
forms the minor product ✔✔Tertiary carbocation has more alkyl groups attached
Positive inductive effect
Reduce + charge on carbon
More stable
Remains in reaction mixture for longer so more likely to react with electrophile
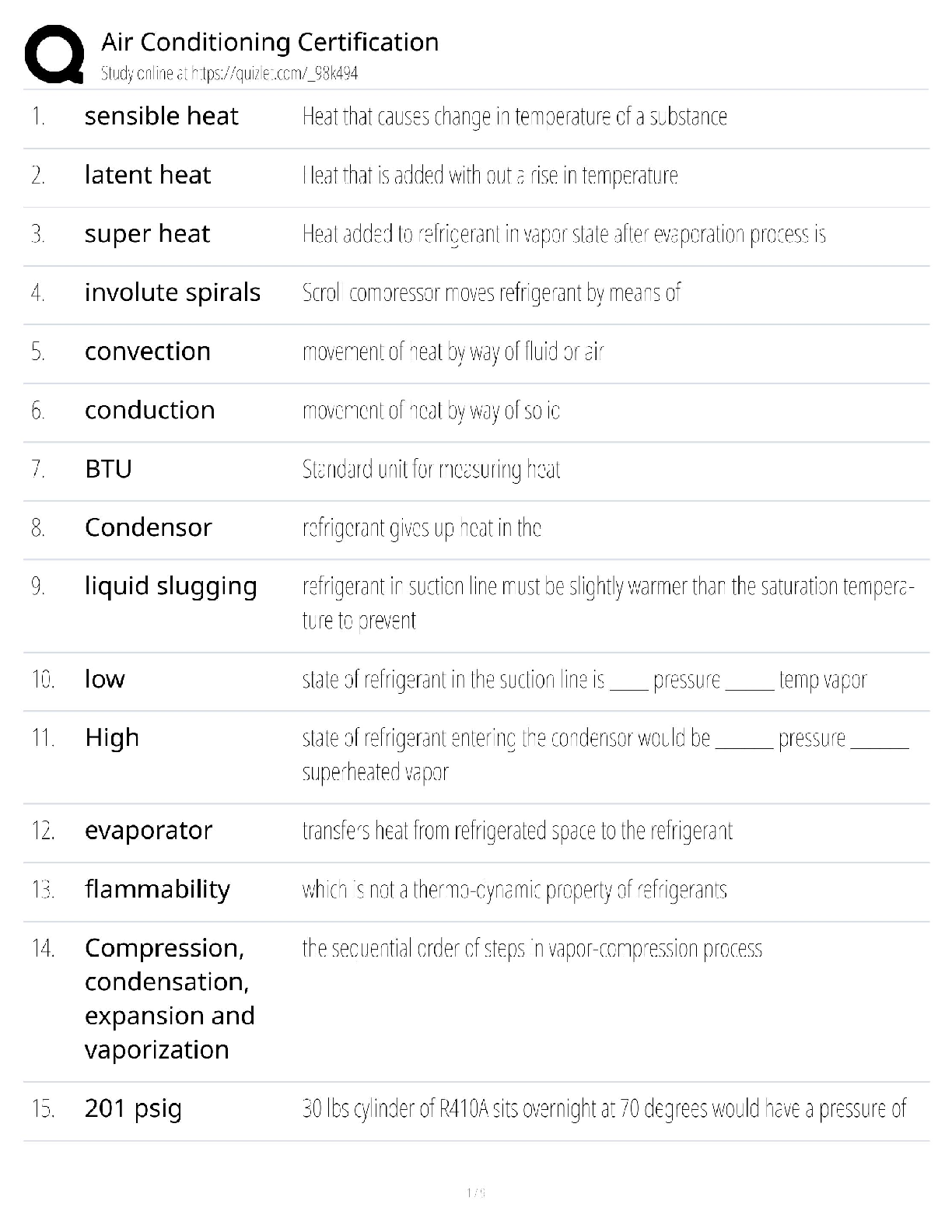
Give a physical properties of polymers ✔✔They have high melting/boiling points
When asked to draw the repeating unit what should you draw? ✔✔Just the atoms and bonds
(single covalent)
There should be no brackets or n
Why are polymers with very few branches strong and hard? ✔✔Very compact
Chains can pack together
van der Waals' strong
Why are branched polymers weaker and softer? ✔✔The polymer chains cannot pack closely
together
van der Waals' between chains weaker
What is an electrophile? ✔✔A substance which can accept a pair of electrons
(electron pair acceptor)
What is a carbocation? ✔✔A species which contains a positive charge on a carbon atom
Describe the mechanism by which HBr is added to ethene ✔✔e-'s move from area of high
electron density to delta + H
e-'s in H-Br bond move to delta - Br
Carbocation forms
Lone pair of e-'s on :Br- attracted to C+ HBr has been added across the double bond
What key phrase is used to describe how the C=C bond is broken by electrophiles?
✔✔Electrophile has been added across the double bond
What is the product of the addition of H2SO4 to an alkene called? ✔✔alkylhydrogensulfate
How is Br2 reacted with alkenes? ✔✔Br2 gas bubbles through alkene
Absence of light
Temperatures below room temp
Describe the test for unsaturation ✔✔Bromine added to water to form bromine water
Bromine water mixed with organic sample
If colour changes from orange to colourless (without gas released) there are C=C bonds present
If remains orange, there are no C=C bonds
Knowledge and understanding of the production and properties of polymers has developed over
time ✔✔Knowledge and understanding of the production and properties of polymers has
developed over time
Put the carbocations in order of stability ✔✔primary< secondary < tertiary
Explain when major and minor products form in electrophilic addition to unsymmetrical alkenes
✔✔Major product formed via carbocation which has most alkyl groups bonded to it Minor
product is formed via carbocation which has least alkyl groups bonded to it Tertiary carbocation
more stable than secondary more stable that primary
How does the electrophile form in non-polar molecules such as Br2? ✔✔When molecule moves
close area of high electron density (C=C double bond)
It becomes polarised
Electrons in C=C repel electrons in neutral molecule
Forms polar electrophile
True or False
The electrophilic addition reactions of HBr or conc H2SO4 and alkenes occur below room
temperature ✔✔True
And at a good rate for HBr!
True or False
Dilute sulphuric acid is an electrophile ✔✔False
Concentrated sulphuric acid is an electrophile
Why are alkenes vulnerable to electrophilic attack? ✔✔Contain C=C double bond
Area of high electron density
If the monomer is 2-chloropropene, what is the polymer? ✔✔poly(2-chloropropene)
What is an addition reaction? ✔✔A reaction in which two molecules join together to make one
larger molecule
What is heterolytic fission? ✔✔Covalent bond breaks and both electrons move to one atom.
Two oppositely charged ions are formed
[Show More]