Chemistry > QUESTIONS & ANSWERS > AQA A-Level Chemistry (new spec) 1.1 Atomic Structure (2022/2023) Already Passed (All)
AQA A-Level Chemistry (new spec) 1.1 Atomic Structure (2022/2023) Already Passed
Document Content and Description Below
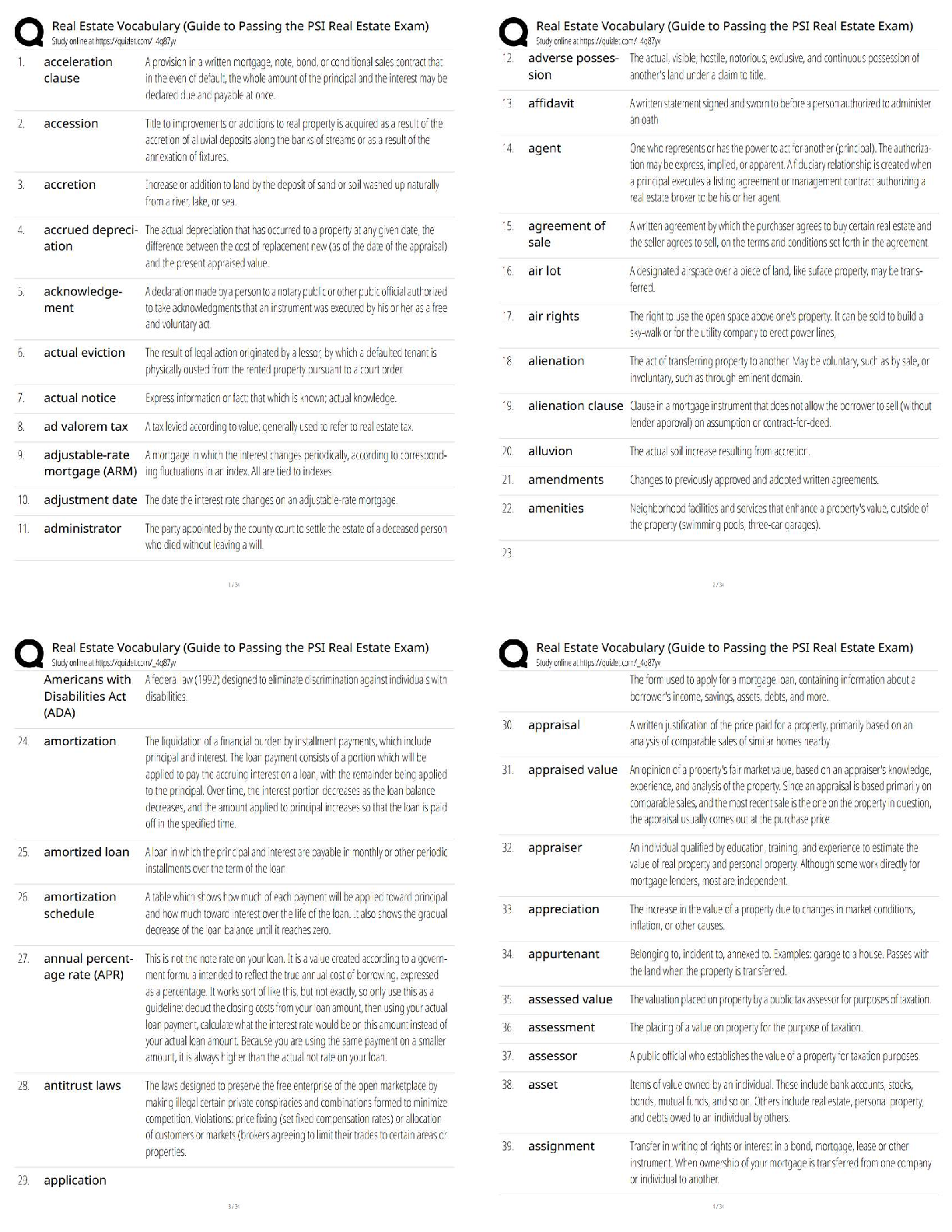
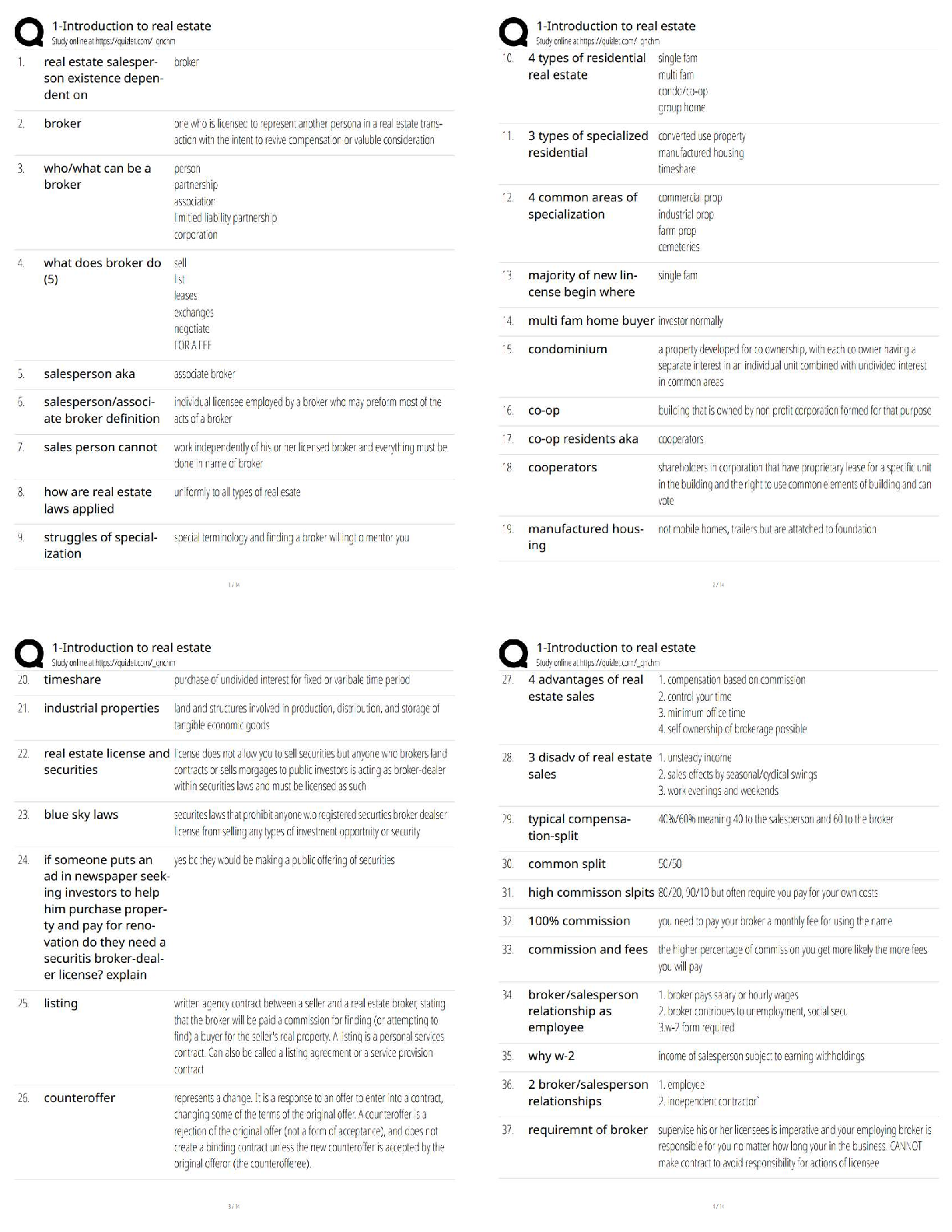
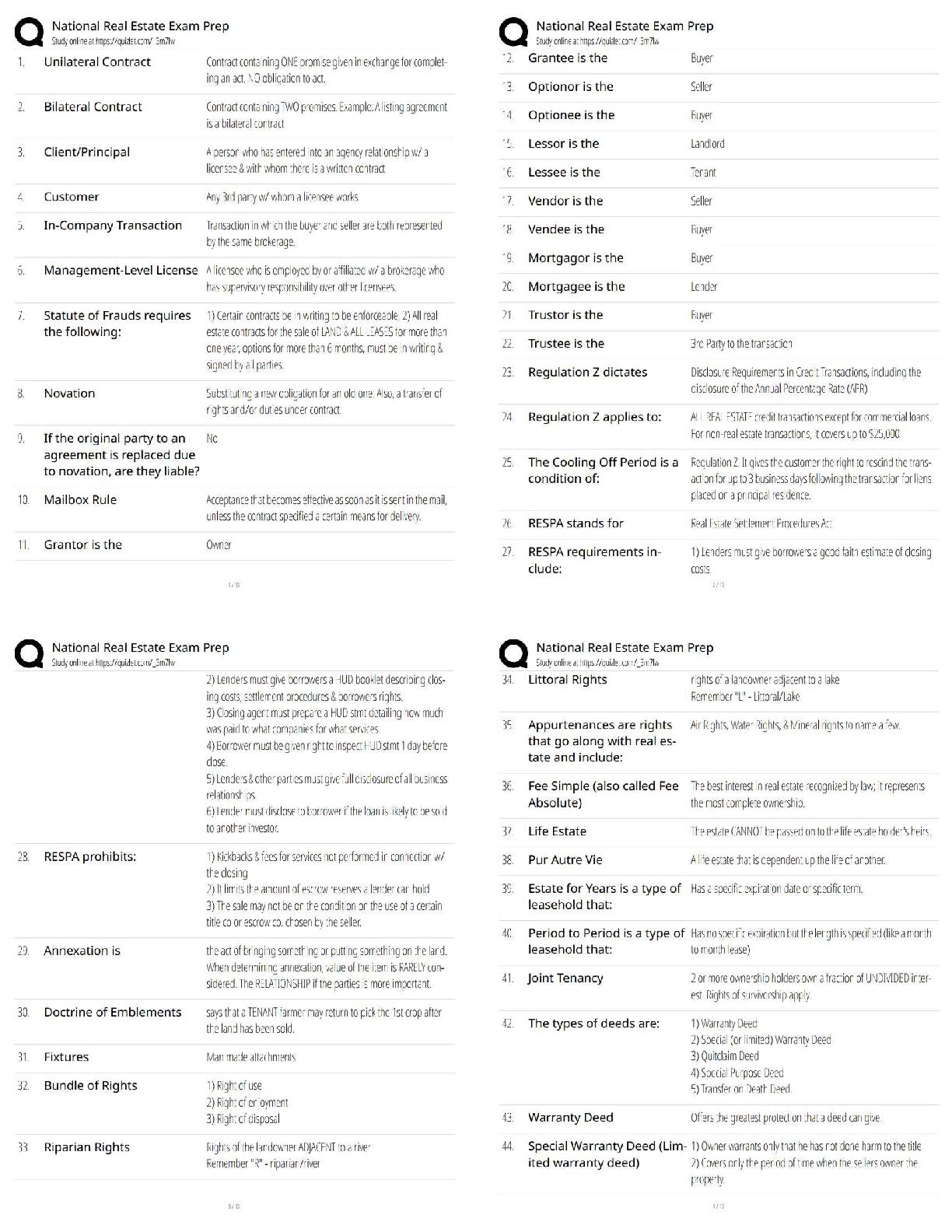
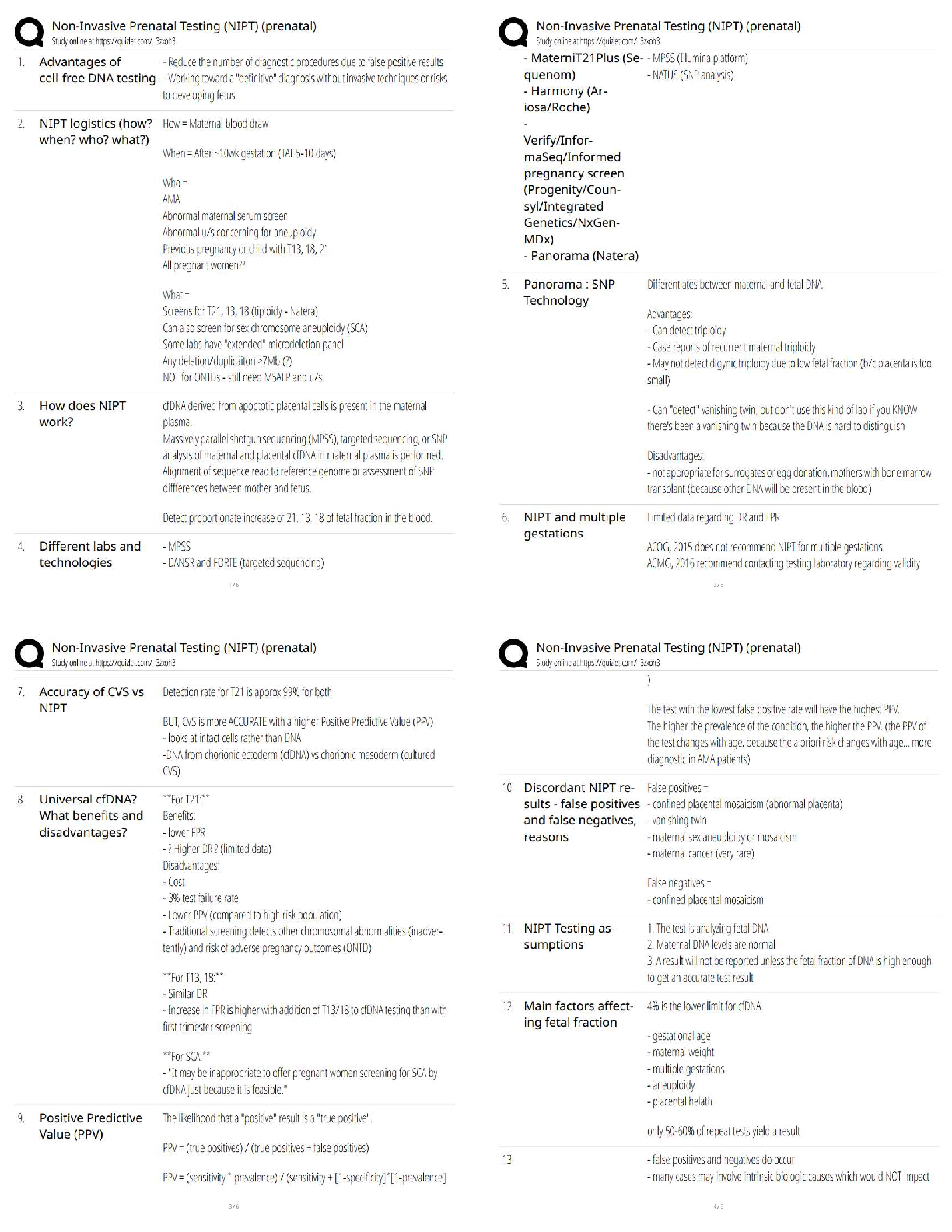
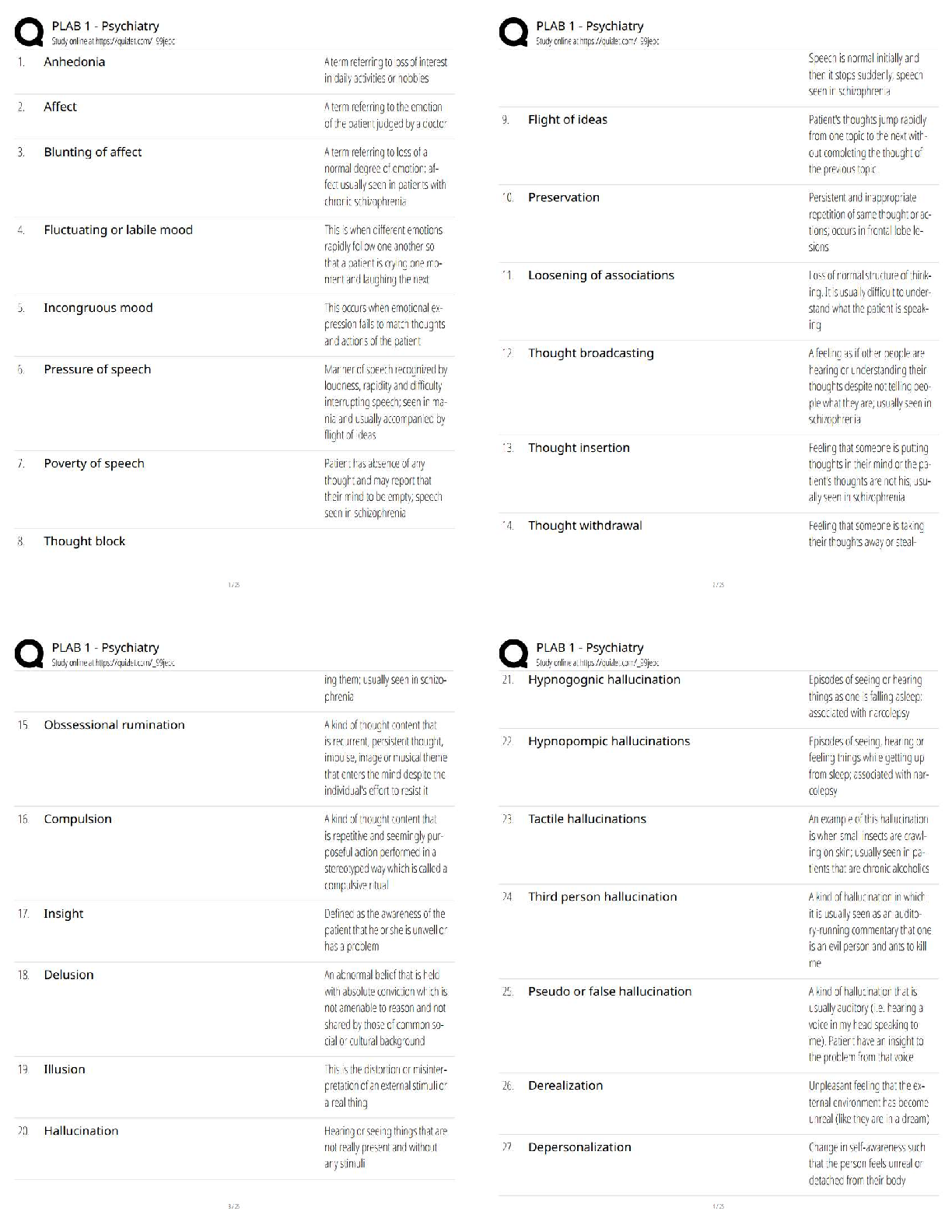
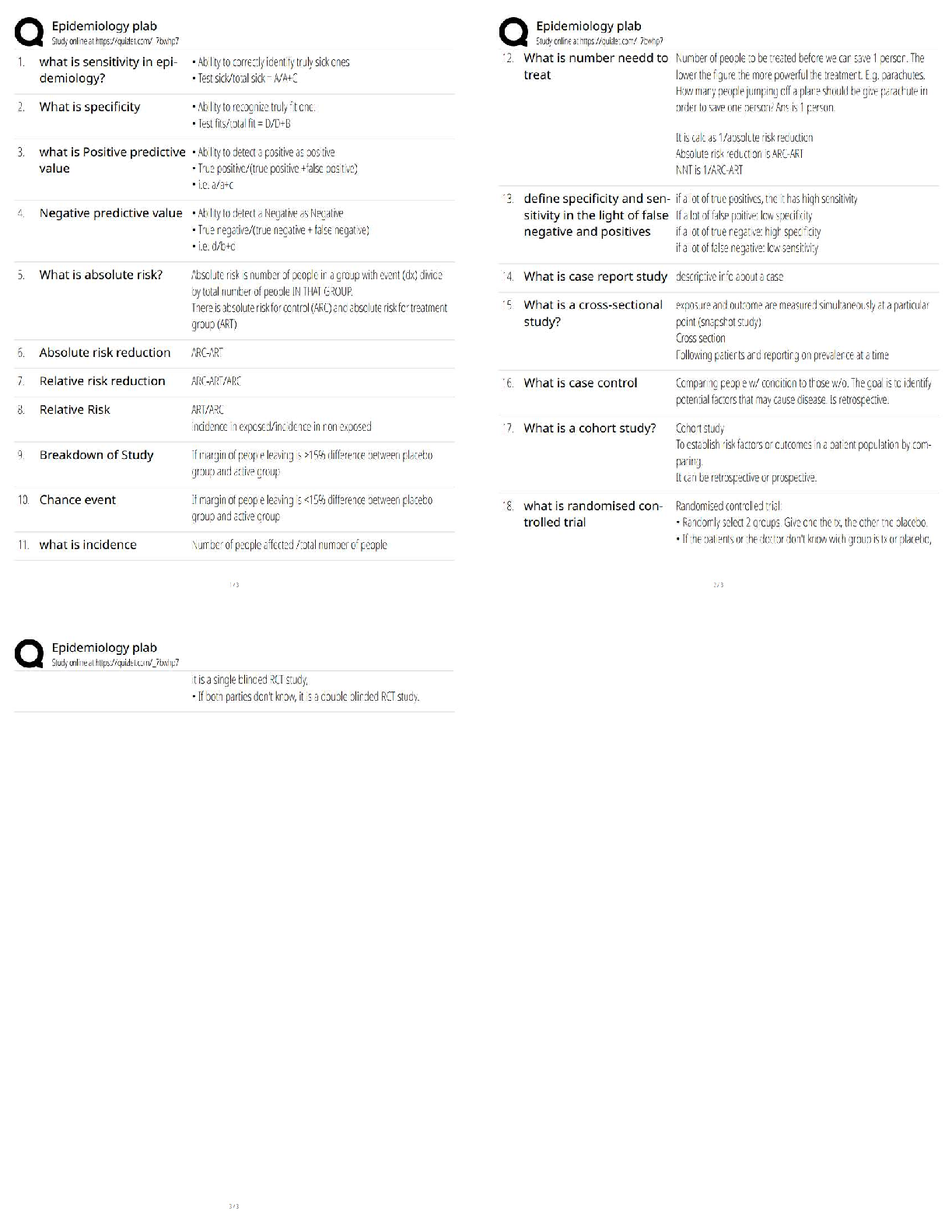
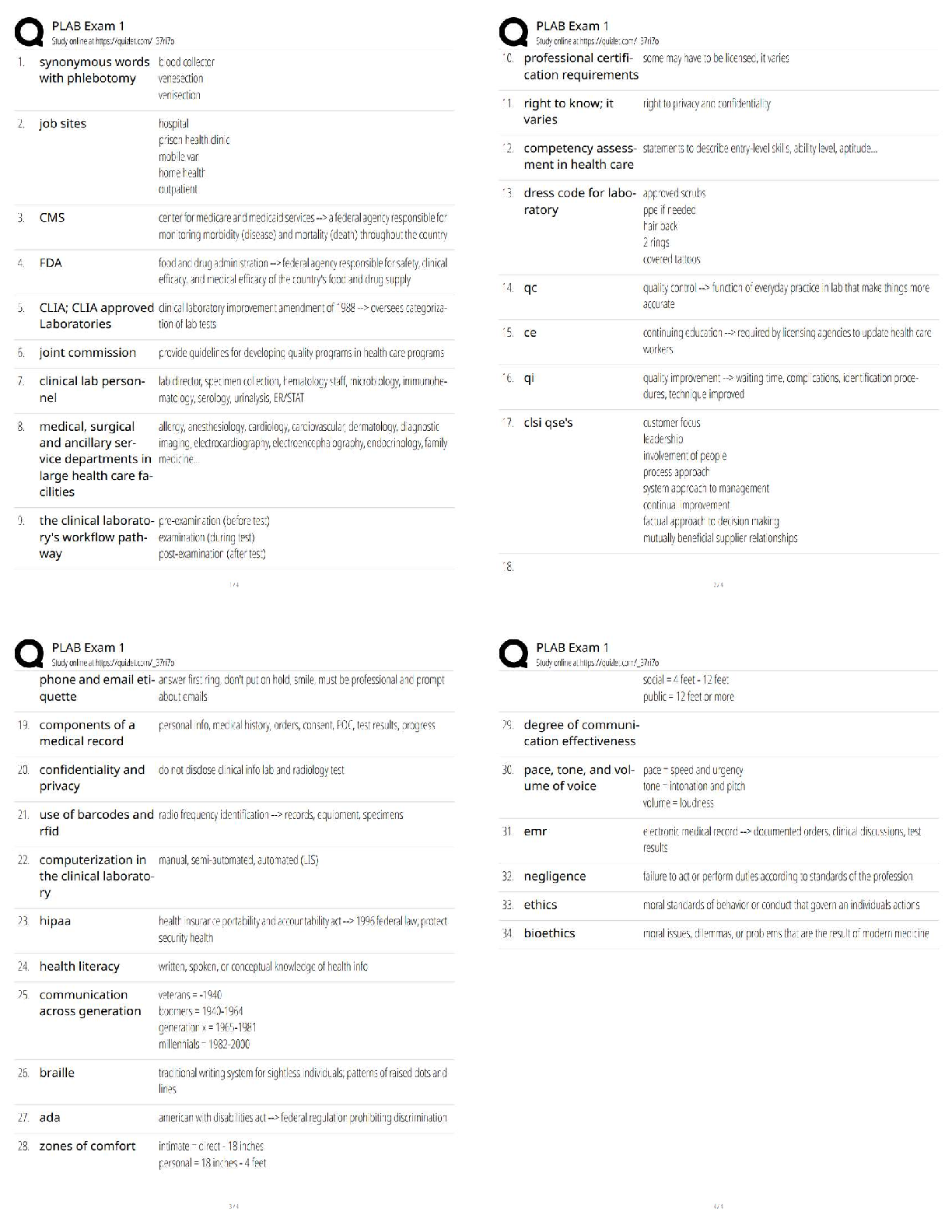
AQA A-Level Chemistry (new spec) 1.1 Atomic Structure (2022/2023) Already Passed What are nucleons? ✔✔Nucleons are protons and neutrons in the nucleus. What is the position of protons? ✔✔N ... ucleus What is the relative mass of a proton ✔✔1 What is the relative charge of a proton? ✔✔+1 What is the position of neutrons? ✔✔Nucleus What is the relative mass of a neutron? ✔✔1 What is the relative charge of a neutron? ✔✔0 What is the position of electrons? ✔✔Orbitals What is the relative mass of an electron? ✔✔1/1800 What is the relative charge of an electron? ✔✔-1 Define mass number ✔✔Sum of protons and neutrons Define atomic number ✔✔The amount of protons in the nucleus Number of neutrons is worked out by... ✔✔Subtracting the atomic number from the mass number Define isotopes ✔✔Atoms of the same element with the same number of protons but a different amount of neutrons Isotopes have similar chemical properties because... ✔✔they have the same electron configuration Isotopes have varying physical properties because... ✔✔they have different masses Define ions ✔✔A charged particle formed when an atom or molecule gains or loses one or more electrons Define Relative Isotopic Mass ✔✔The mass of an atom of a particular isotope compared to 1/12 the mass of an atom of carbon-12 Define Relative Atomic Mass (Ar) ✔✔The average mass of an atom compared to 1/12 the mass of an atom of carbon-12 Define Relative Molecular Mass (Mr) ✔✔The average mass of a molecule compared to 1/12 the mass of an atom of carbon-12 Mass Spectrometer: Why is it kept in vacuum conditions? ✔✔To prevents the ions produced colliding with molecules of air Mass Spectrometer: What happens during ionisation? ✔✔The sample is dissolved in a volatile solvent and forced through a fine, hollow needle which is connected to a positive terminal of a high voltage supply. The sample is shot with an electron gun, which knocks off an electron, producing positively charged ions. Mass Spectrometer: What happens during acceleration? ✔✔Positive ions are attracted to a negatively charged plate, causing them to accelerate. Lighter ions with a higher charge move faster Mass Spectrometer: What happens during ion drift? ✔✔The ions pass through a hole in the negatively charged plate, forming a beam, which drift towards the detector Mass Spectrometer: What happens during detection? ✔✔The lighter ions which have the fastest velocities arrive at the detector first. The positive ions pick up an electron, causing a current to flow Mass Spectrometer: What happens during data analysis? ✔✔Signals from the detector are passed to a computer which generates a mass spectrum Mass Spectrometer: What is the mass spectrometer measuring? ✔✔M:Z ratio and abundance How do you calculate R.A.M from a mass spectrum? ✔✔You multiply the m:z ratio and abundance together for each peak. You then add the totals together and divide by the total relative abundance. What sub-level can principle level 1 hold? ✔✔1s What sub-levels can principle level 2 hold? ✔✔2s, 2p What sub-levels can principle level 3 hold? ✔✔3s, 3p, 3d What sub-levels can principle level 4 hold? ✔✔4s, 4p, 4d, 4f How many electrons can sub-level S hold? ✔✔2 How many electrons can sub-level P hold? ✔✔6 How many electrons can sub-level D hold? ✔✔10 How many electrons can sub-level F hold? ✔✔14 What shape are S sub-levels? ✔✔Spherical What shape are P sub-levels? ✔✔Dumbbells Why is the 3d sub-level filled after the 4s sub-level? ✔✔Because 3d is higher in energy, so 4s is filled first and then when 3d is filled, the energy level drops to below 4s First Ionisation Energy ✔✔The enthalpy change when one mole of gaseous atoms forms one mole of gaseous atoms with a singe positive charge by losing one electron Second Ionisation Energy ✔✔The enthalpy change when one mole of gaseous ions with a single positive charge forms one mole of gaseous ions with a 2+ charge by losing one electron Factors affect ionisation energy: Nuclear Charge ✔✔The higher the nuclear charge (the more protons there are) the greater the attraction of the outer electrons to the nucleus Factors affect ionisation energy: The distance of the outer electrons to the nucleus ✔✔The larger the atom, the further the outer electrons are away from the nucleus, making the attraction weaker Factors affect ionisation energy: Shielding ✔✔Electrons in the outer shell are repelled by electrons in complete inner shells, weakening the attraction of the nucleus Factors affect ionisation energy: In-orbital Repulsion ✔✔Once the electrons are paired, they start repelling each other, the degree of repulsion affects how easy it is to remove electrons Why are the successive ionisation energies always higher? ✔✔Because a positive ion is formed from the first ionisation energy, meaning the nuclear attraction increases, making it harder for the second electron to be removed Why is He's ionisation energy bigger than H's? ✔✔-Increased nuclear charge (gone from + to +2) -Same shielding -Same distance from nucleus to outer electron Why is there such a large drop of ionisation energy between He and Li? ✔✔-Increased shielding (Li enters 2s orbital, He still on 1s) -Increased nuclear charge -Outer electrons are further away from the nucleus Why is Be's ionisation energy greater than Li? ✔✔-Increased nuclear charge -Shielding same as Li -Outer electrons are the same distance away from the nucleus in both Why is B's ionisation energy higher than Be? ✔✔-Increased nuclear charge -Increased shielding (B enters 2p orbital) so is shielded by 1s and 2s orbitals -Outer electrons are further away in B Why is there a drop of ionisation energy between N and O? ✔✔-Increased nuclear charge -Shielding same (still in 2p orbital) -Same amount of shielding -In orbital repulsion as the electron is entering a partially filled p sub-level [Show More]
Last updated: 2 years ago
Preview 1 out of 8 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Also available in bundle (1)
Click Below to Access Bundle(s)

AQA A LEVEL Chemistry Bundled Exams Questions and Answers 100% Pass
AQA A LEVEL Chemistry Bundled Exams Questions and Answers 100% Pass
By Nutmegs 2 years ago
$22
13
Reviews( 0 )
$10.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
May 07, 2023
Number of pages
8
Written in
All
Additional information
This document has been written for:
Uploaded
May 07, 2023
Downloads
0
Views
151










.png)