AQA > A-Level Question Paper > AQA A level CHEMISTRY Paper 3 JUNE 2022 FINAL QUESTION PAPER. (All)
AQA A level CHEMISTRY Paper 3 JUNE 2022 FINAL QUESTION PAPER.
Document Content and Description Below
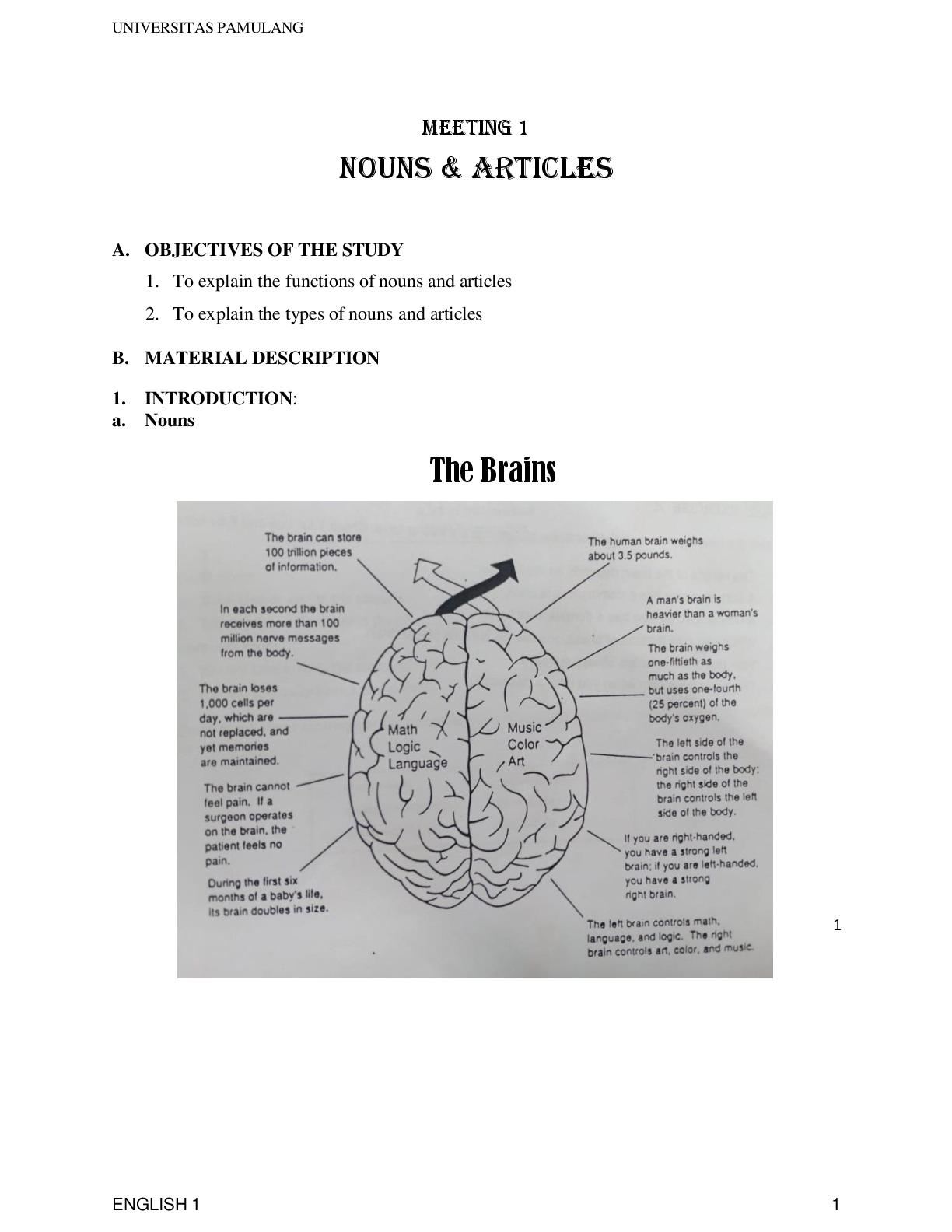
A level CHEMISTRY Paper 3 JUNE 2022 Time allowed: 2 hours Materials For this paper you must have: • the Periodic Table/Data Booklet, provided as an insert (enclosed) • a ruler with mill ... imetre measurements • a scientific calculator, which you are expected to use where appropriate. Instructions • Use black ink or black ball-point pen. • Fill in the boxes at the top of this page. • Answer all questions. • You must answer the questions in the spaces provided. Do not write outside the box around each page or on blank pages. • If you need extra space for your answer(s), use the lined pages at the end of this book. Write the question number against your answer(s). • All working must be shown. • Do all rough work in this book. Cross through any work you do not want to be marked. Information • The marks for questions are shown in brackets. • The maximum mark for this paper is 90. Advice • You are advised to spend 70 minutes on Section A and 50 minutes on Section B. A LEVEL CHEMISTRY 2022 PAPER 3 There are no questions printed on this page DO NOT WRITE ON THIS PAGE ANSWER IN THE SPACES PROVIDED box Section A box Answer all questions in this section. A value for Answer enthalpy of solution can be determined in two ways: • from a cycle, using lattice enthalpy and enthalpies of hydration • from the results of a calorimetry experiment. . Define the term enthalpy of lattice dissociation. [2 marks] . The enthalpy of solution for ammonium nitrate is the enthalpy change for the reaction shown. NH4NO3(s) + aq → NH4(aq) + NO (aq) H = +26 kJ mol1 Table 1 NH4 (g) NO3 (g) Enthalpy of hydration hydH / kJ mol 1 307 314 Draw a suitably labelled cycle and use it, with data from Table 1, to calculate the enthalpy of lattice dissociation for ammonium nitrate. [3 marks] Enthalpy of lattice dissociation kJ mol1 Turn over ► . A student does an experiment to determine a value for the enthalpy of solution for ammonium nitrate. The student uses this method. • Measure 25.0 cm3 of distilled water in a measuring cylinder. • Pour the water into a beaker. • Record the temperature of the water in the beaker. • Add 4.00 g of solid NH4NO3 to the water in the beaker. • Stir the solution and record the lowest temperature reached. Table 2 shows the student’s results. Table 2 Initial temperature / °C 20.2 Lowest temperature / °C 12.2 Calculate the enthalpy of solution, in kJ mol1, for ammonium nitrate in this experiment. Assume that the specific heat capacity of the solution, c = 4.18 J K1 g1 box Assume that the density of the solution = 1.00 g cm3 [3 marks] Enthalpy of solution kJ mol1 [Show More]
Last updated: 2 years ago
Preview 1 out of 44 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Also available in bundle (2)
Click Below to Access Bundle(s)

AQA A LEVEL CHEMISTRY PAPER 3 JUNE 2022 FINAL QUESTION PAPERS AND MARK SCHEMES
AQA A LEVEL CHEMISTRY PAPER 3 JUNE 2022 FINAL QUESTION PAPERS AND MARK SCHEMES
By examstore001 2 years ago
$28
2

AQA A LEVEL CHEMISTRY PAPER 1 ,2 AND 3 JUNE 2022 FINAL QUESTION PAPERS AND MARK SCHEMES
AQA A LEVEL CHEMISTRY PAPER 1 ,2 AND 3 JUNE 2022 FINAL QUESTION PAPERS AND MARK SCHEMES
By examstore001 2 years ago
$80
6
Reviews( 0 )
$17.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
May 22, 2023
Number of pages
44
Written in
All
Additional information
This document has been written for:
Uploaded
May 22, 2023
Downloads
0
Views
125