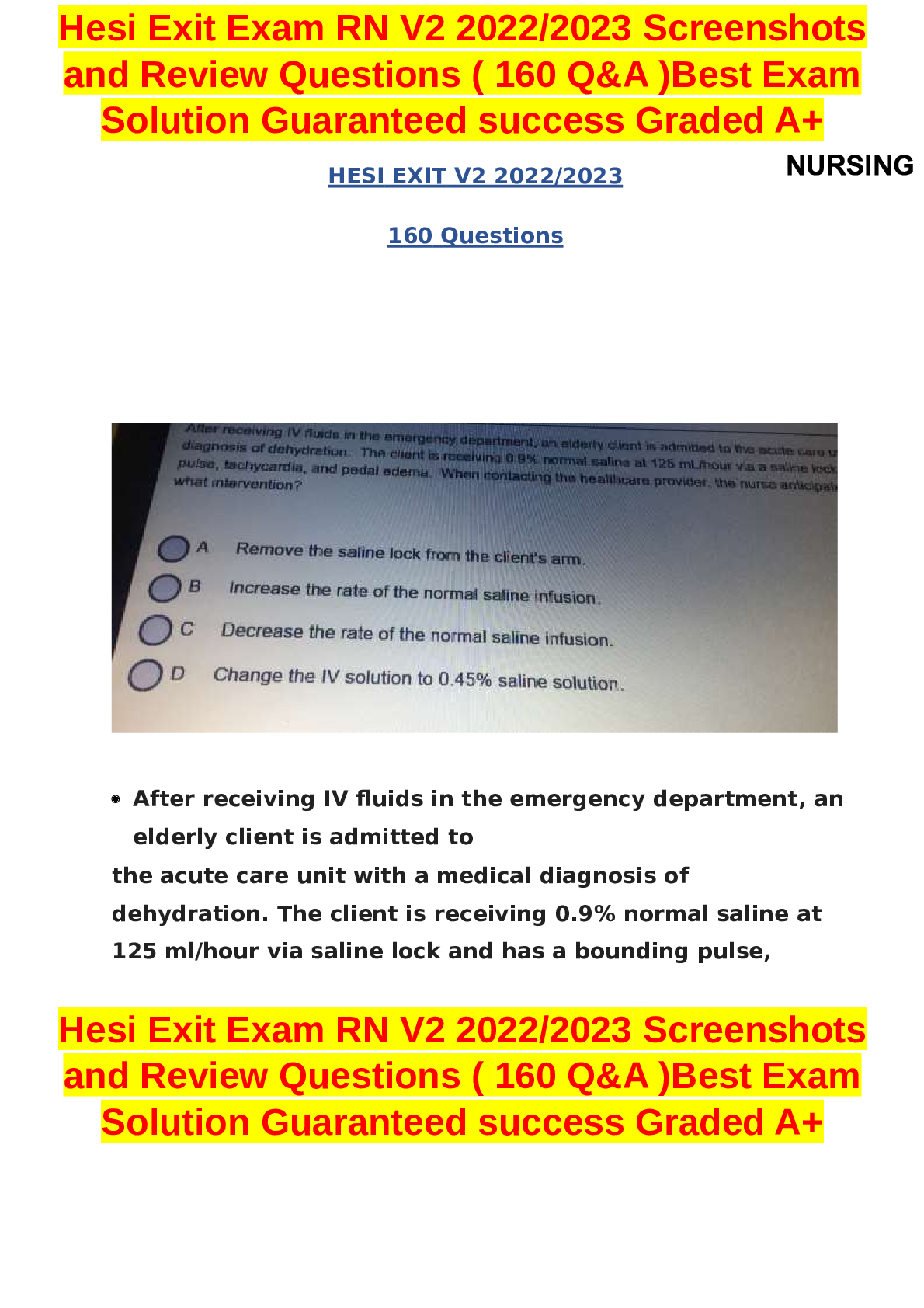
NUR 641E final Exam Advanced for the
Nurse Educator with Complete Solutions. VERIFIED
Pharmacokinetics involves - ANSWER-absorption, distribution, metabolism and
elimination).
Absorption: - ANSWER-absorption from the
...
NUR 641E final Exam Advanced for the
Nurse Educator with Complete Solutions. VERIFIED
Pharmacokinetics involves - ANSWER-absorption, distribution, metabolism and
elimination).
Absorption: - ANSWER-absorption from the administration site either directly or
indirectly into the blood/plasma.
Distribution: - ANSWER-reversibly or irreversibly move from the bloodstream into the
interstitial and intracellular
Metabolism: - ANSWER-biotransformed via hepatic metabolism or by other tissues.
Elimination: - ANSWER-tissues. lastly, the drug and its metabolites are eliminated from
the body.
route of administration with the highest bioavailability - ANSWER-intravenous; putting
entire dose into a patient's vein and bypassing absorption.
avoids first-pass metabolism - ANSWER-Intravenous route
administration has variable and erratic absorption. n - ANSWER-Rectal administration
4. Steady state (SS) - ANSWER-absorption. n is usually reached within 4-5 half-lives of
drug.
Half-life of a drug is - ANSWER-how long it takes for half the drug to be excreted from
the body. Determines how frequently the drug must be administered. Predicts how long
toxic effects can last.is constant with first-order pharmacokinetics of a drug.
Zero-order (nonlinear) pharmacokinetics - ANSWER-means a drug is metabolized at a
constant rate per unit time.
CYP3A4 substrate drugs - ANSWER-may have enhanced activity if any CYP3A4
inducer drugs are used along with it.
Drug development process involves these steps according to the FDA: - ANSWERDiscovery: laboratory research to develop the new drug. Preclinical research with
animal testing for safety (Phase I). Clinical research on human subjects for medication
safety (Phase II). Clinical research in humans comparing the new drug to accepted
medications placebo depending on the study (Phase III). FDA review of the results to
determine approval. Post marketing study to identify adverse effects not found in earlier
clinical studies (Phase IV)
NURSING
2. Medication safety organizations - ANSWER-The Institute for Safe Medication
Practices (ISMP) The Institute of Medicine (IOM) The Joint Commission The National
Coordinating Council for Medication Error Reporting and Prevention (NCC MERP) Food
and Drug Administration (FDA) Safe Use Initiative
Two basic type of ADRS: - ANSWER-pharmacological and idiosyncratic.
85% to 90% of ADRS - ANSWER-are pharmacological.
Adverse drug reactions are usually preventable, - ANSWER-frequently occur in a
hospital or nursing home setting, and include medication errors, adverse drug effects,
and allergic idiosyncratic type reactions.
ADRS are not commonly reported; - ANSWER-the FDA does not mandate that ADRS
be reported.
Polypharmacy - ANSWER-involves using multiple health care providers for care, using
multiple medications, and using several pharmacies prescription filling.
Angiotensin converting enzyme inhibitors (ACEIS): - ANSWER-lisinopril, captopril,
enalapril, ramipril, benazepril, fosinopril.
ACEIS reduce blood pressure enzyme. - ANSWER-by suppressing the release of
angiotensin-converting enzyme.
Important side effects of ACE inhibitors - ANSWER-Important include cough and
angioedema; discontinue the ACEI if angioedema occurs.
Angiotensin II receptor blocking agents (ARBS): - ANSWER-Icandesartan (Atacand),
eprosartan (Teveten), irbesartan (Avapro), losartan (Cozaar), telmisartan (Micardis) and
valsartan (Diovan).
ARBS reduce blood pressure - ANSWER-by blocking angiotensin II receptors.
Essential (primary) hypertension - ANSWER-Essential (primary) accounts for 90% of
cases; secondary hypertension may caused by chronic renal failure.
Nitroglycerin - ANSWER-Nitroglycerin is a nitrate drug that can be administered IV, SL,
a topical ointment and as a transdermal patch.
Nitrates are contraindicated - ANSWER-with PDE-5 inhibitors (e.g., sildenafil and
vardenafil)
Amiodarone is the antiarrhythmic - ANSWER-Of choice when there is coexisting heart
failure; can cause thyroid and pulmonary toxicity.
[Show More]