CAPP Biology Test 1
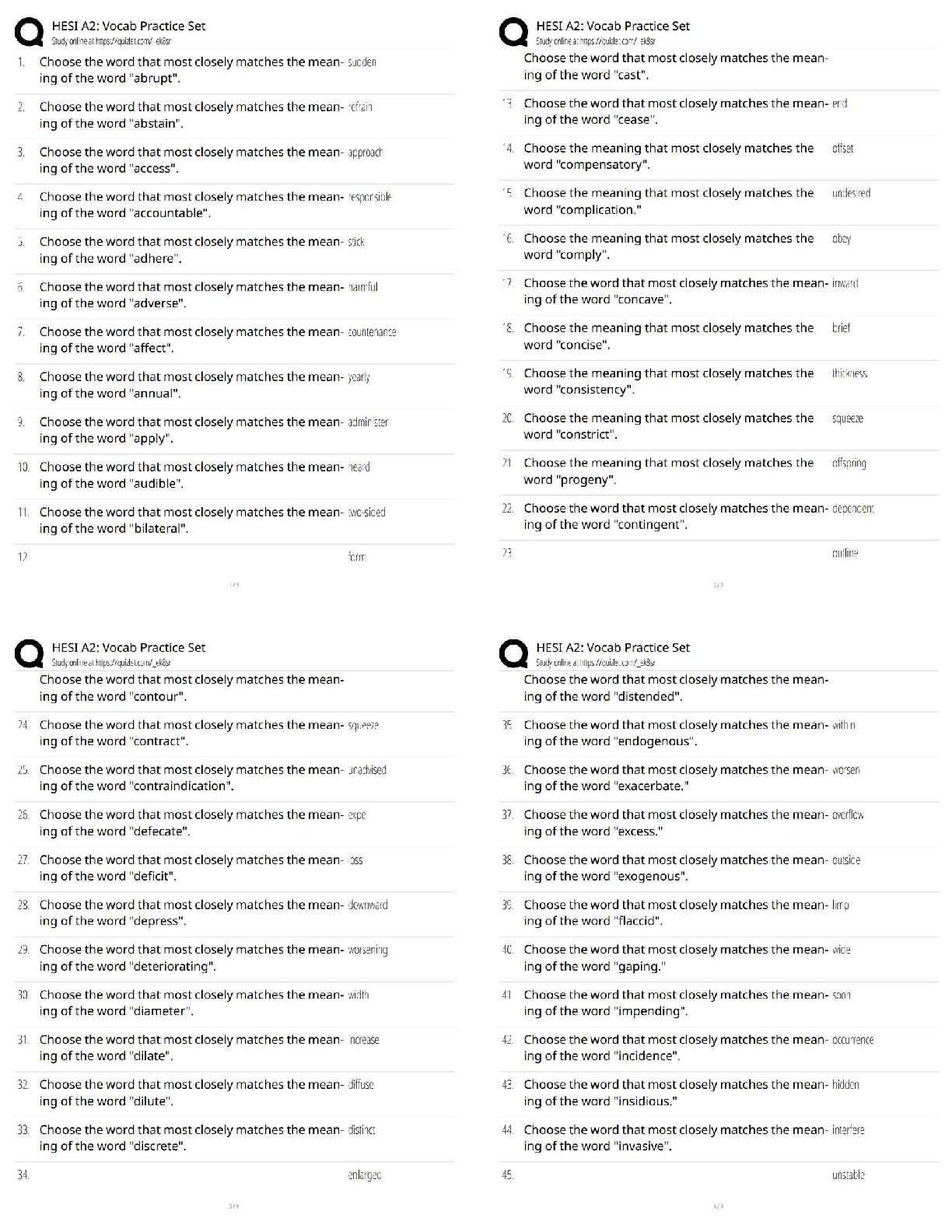
Why is it impossible for humans to digest food that contains cellulose?
a - the acidic environment in the human stomach makes it impossible to break the bonds in
cellulose
b - human digestive enzy
...
CAPP Biology Test 1
Why is it impossible for humans to digest food that contains cellulose?
a - the acidic environment in the human stomach makes it impossible to break the bonds in
cellulose
b - human digestive enzymes cannot break down the beta - 1,4 glycosidic linkage in cellulose,
which requires a special enzyme that is absent in humans
c - there is no energy available in fiber
d - an inactive form of cellulase in the human digestive system tract renders it undigested and
removes it as waste - ✔✔b - Human digestive enzymes cannot break down the beta - 1,4
glycosidic linkage in cellulose, which requires a special enzyme that is absent in humans
Which category of lipid would be used to produce sex hormones like estradiol and testosterone
fat in humans?
a - wax
b - steroid
c - triglyceride
d - phospholipid - ✔✔steroid
Which category of liid would be used to store body fat in humans?
a - wax
b - steroid
c - triglyceride
d - phospholipid - ✔✔triglyceride
Which category of lipid would be used to build plasma membranes in cells?
a - wax
b - steroid
c - triglyceride
d - phospholipid - ✔✔phospholipid
What categories of amino acids would you expect to find on the surface of a water-soluble
protein and which would you expect to find on the interior?
a - positively charged and negatively charged on the surface and polar and nonpolar on the
interior
b - polar and nonpolar on the surface and positively charged and negatively charged on the
interior
c - polar and charged on the surface and nonpolar on the interior
d - nonpolar and charged on the surface and polar on the interior - ✔✔polar and charged amino
acids will be found on the interior
Where is the linkage made that combines two amino acids in primary protein structure?
a - between the R group of one amino acid and the R group of the second
b - between the carboxyl group of one and the amino group of the other
c - between the 6 carbon of both amino acids
d - between the nitrogen atoms of the amino groups in the amino acids - ✔✔b - between the
carboxyl group of one and the amino group of the other
Which of the following would yield a positive result using a biuret test?
a - all of the following options
b - a solution of water and lysine amino acid monomers
c - a solution of water, lysine amino acid monomers, and arginine amino acid monomers
d - a solution of water and a water-soluble polypeptide - ✔✔d - a solution of water and a watersoluble polypeptide
The alpha helix and the beta pleated sheet are part of which protein structure?
a - the primary structure
b - the secondary structure
c - the tertiary structure
d - the quaternary structure - ✔✔b - the secondary structure
What type of protein facilitates or accelerates chemical reactions?
a - membrane transport protein
b - tRNA molecule
c - an enzyme
d - a hormone - ✔✔c - an enzyme
What is a suggested and testable explanation for an event called?
a - scientific method
b - theory
c - discovery
d - hypothesis - ✔✔d - hypothesis
What is the name for the formal process through which scientific research is checked for
originality, significance, and quality before being accepted into scientific literature?
a - peer review
b - the scientific method
c - publication
d - public speaking - ✔✔a - peer review
What happens to the pH of a solution when acids are added?
a - stays the same
b - increases
c - decreases
d - increases then decreases - ✔✔c - decreases
Why do hydrogen and oxygen form polar covalent bonds within water molecules?
a - oxygen is more electronegative, generating a partial negative charge near the oxygen atoms
b - hydrogen is more eneg, partial neg near hydrogen
c - oxygen is more eneg, partial pos near oxygen
d - hydrogen is more eneg, partial positive near hydrogen - ✔✔a - a - oxygen is more
electronegative, generating a partial negative charge near the oxygen atoms
Which of the following statements is not true?
a - water is the most abundant molecule in the earth's atmosphere
b - water is essential for life
c - water can stabilize the temperature of nearby air
d - water is polar - ✔✔a - water is the most abundant molecule in the earth's atmosphere
Which of the following statements is true?
a - acids and bases cannot mix together
b - acids, not bases, can change the pH of a solution
c - acids and bases can neutralize each other
d - acids donate hydroxide ions (OH-) bases donate hydrogen ions (h+) - ✔✔c - acids and bases
can neutralize each other
Surface tension of water is caused by
a - neither adhesion nor cohesion
b - both adhesion and cohesion
c - only adhesion
d - only cohesion - ✔✔d - only cohesion
What chemicals serve to stabilize the pH of internal solutions in organisms?
a - buffers
b - salts
c - acids
d - bases - ✔✔a - buffers
Which of the following would not require a dehydration synthesis reaction?
a - adding fatty acid chains to a glycerol to form a triglyceride
b - combining amino acids using peptide bonds
c - adding nucleotides to DNA
d - combining glucose and fructose into sucrose - ✔✔c - adding nucleotides to DNA
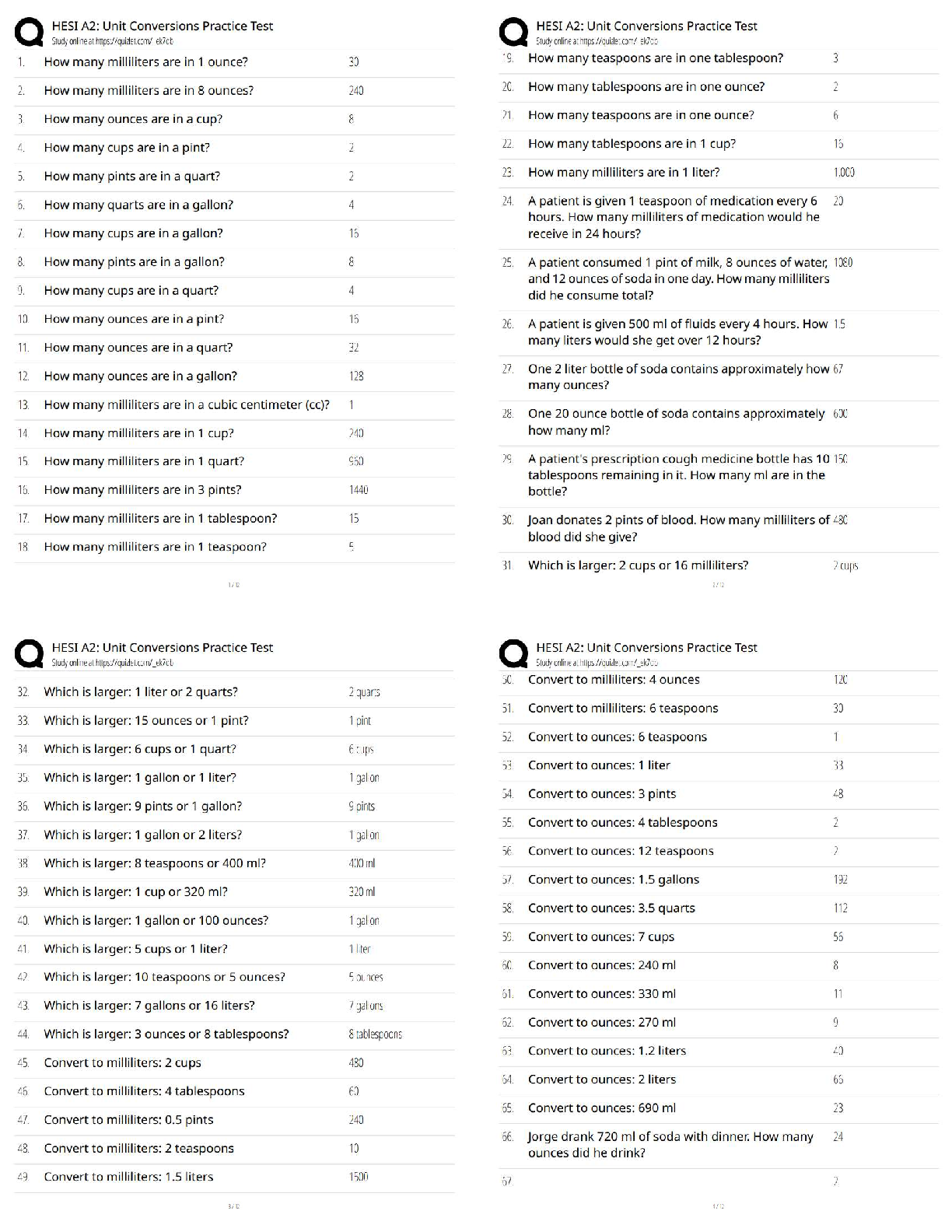
A fat is represented by - ✔✔
A amino acid is represented by - ✔✔
A disaccharide is represented by - ✔✔
The nucleotide is represented by - ✔✔
In dehydration synthesis what molecule would be released as a result of the reaction depicted?
a - water
b - oxygen
c - hydrogen
d - hydroxyl - ✔✔a - water
Energy is released as a result of which of the following types of chemical reactions?
a - hydrolysis
b - peptide bond formation
c - triglyceride formation
d - dehydration synthesis - ✔✔a - hydrolysis
What is removed during the formation of nucleic acid polymers?
a - phosphates
b - amino acids
c - carbon
d - hydroxyl groups - ✔✔a - phosphates
What hormones are made from cholesterol?
a - estradiol and testosterone
b - insulin and growth hormone
c - progesterone and gluucagon
d - prolactin and thyroid hormone - ✔✔a - estradiol and testosterone
Which of the following characteristics is not true for unsaturated fats?
a - they tend to dissolve in water easily
b - their basic structure is a glycerol backbone with attached fatty acid chains
c - they tend to be liquid at room temperature
d - they contain at least one double bond within the fatty acid carbon chain - ✔✔a - they tend to
dissolve in water easily
[Show More]