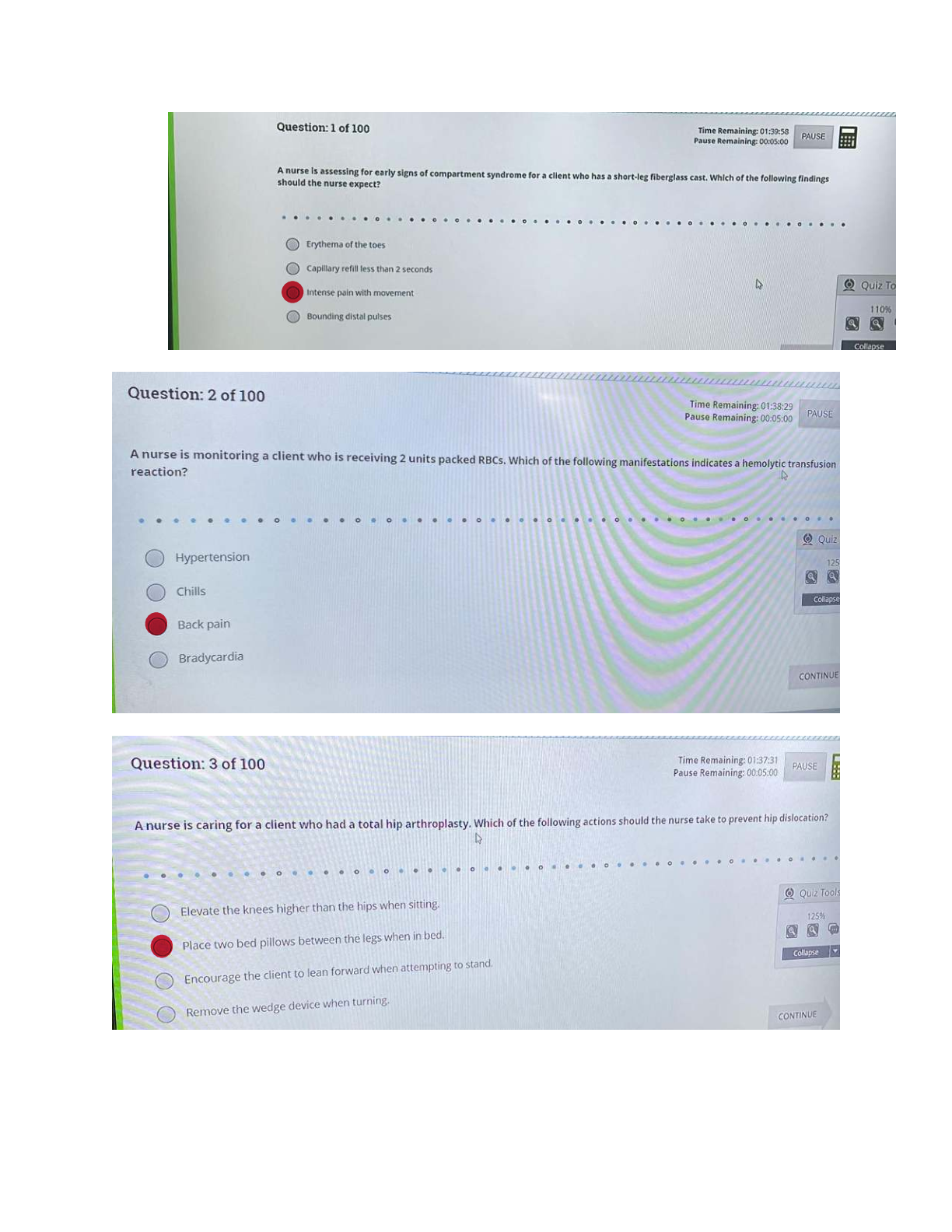
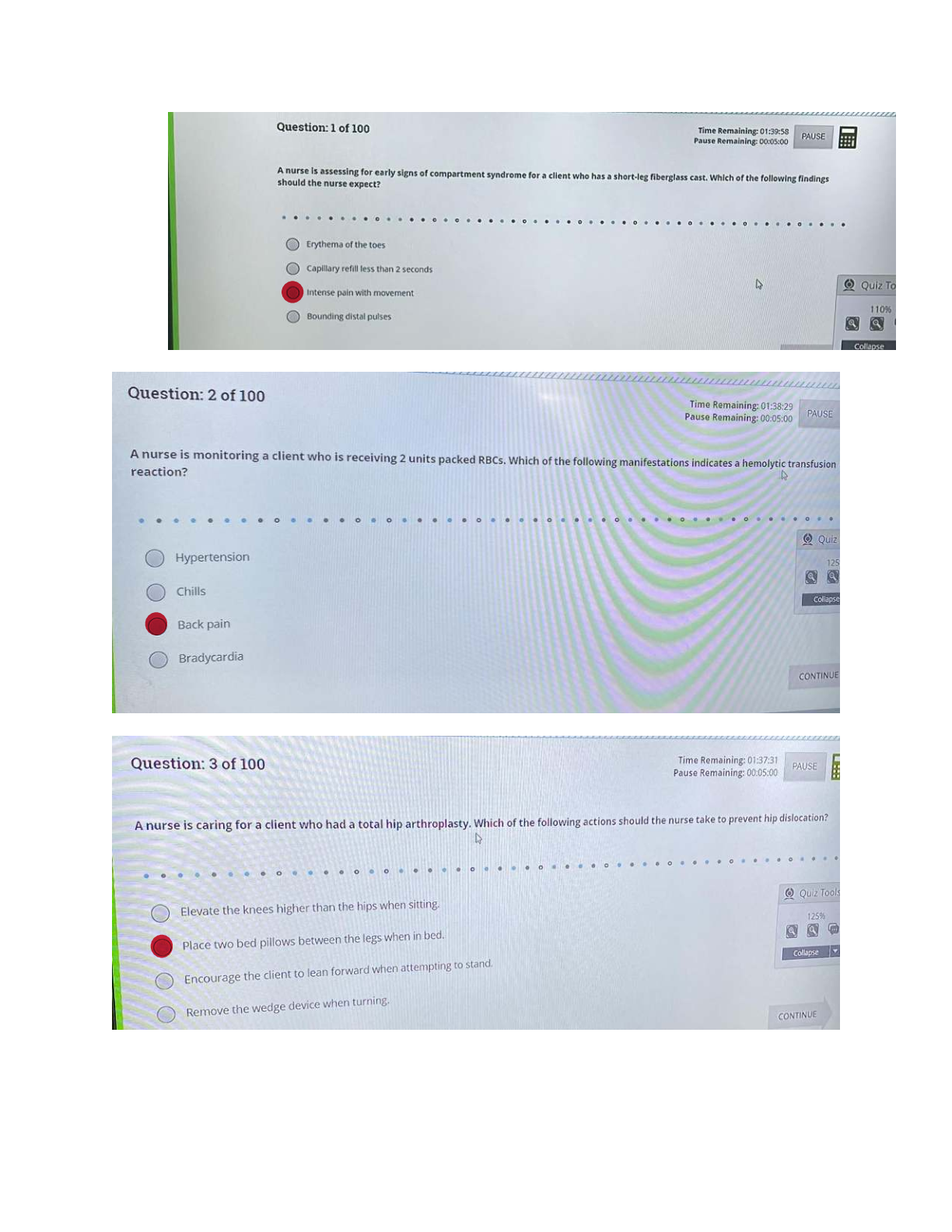
CHEM 104 Experiment 1 Kinetics - Portage Learning Title: Kinetics Purpose: The purpose of the first reaction was to vary the concentrations to observe the relationship of concentration and rate of the reaction. The purp
...
CHEM 104 Experiment 1 Kinetics - Portage Learning Title: Kinetics Purpose: The purpose of the first reaction was to vary the concentrations to observe the relationship of concentration and rate of the reaction. The purpose of the second experiment was also to vary the concentrations of hydrogen peroxide and iodine and determine how the rate was affected based on the concentrations. Order of reaction was also discussed and determined in the first and second reactions. The purpose of the third experiment was to observe how temperature affects the rate of a reaction using hydrogen peroxide and iodine. Procedure: 1. For the first experiment, supplies were gathered, including 5 test tubes labelled 1-5, test tube holder, beaker to measure (mL), water, and 0.1 M S2O3 2- (mL). 2. From the 0.1 M S2O3 2- (mL) solution, 10.0 mL was placed in test tube 1, 8.0 mL was placed in test tube 2, 6.0 mL was placed in test tube 3, 4.0 mL was placed in test tube 4, and 2.0 mL was placed in test tube 5. 3. The concentration (M) was calculated using C1V1=C2V2 for each test tube. 4. Then, we calculated the concentration of the solution with HCl using C1V1=C2V2 for each test tube. 5. Test tube 1 was mixed with 10.0 mL of HCl. A timer was initiated and we observed for changes as it reacted. We expected the solution to become cloudy and opaque. 6. As the reaction occurred, we placed a black piece of paper behind the tube to make it easier to determine the changes occurring. 7. It was determined that test tube 1 was done reacting after 33 seconds. Rate = 1/33 s 8. Test tube 2 was then mixed with 10.0 mL of HCl. A timer was started again. 9. It was determined that test tube 2 was done reacting after 45 seconds. Rate = 1/45 s 10. Test tubes 3, 4, and 5 were mixed with 10.0 mL of HCl and the reactions were timed. 11. For the second experiment, we gathered test tubes, hydrogen peroxide, iodine, gas collection apparatus. 12. In the second experiment we held one part constant, while varying the other half in terms of the concentration.
[Show More]