Chapter 15 Test Questions with Verified Answers
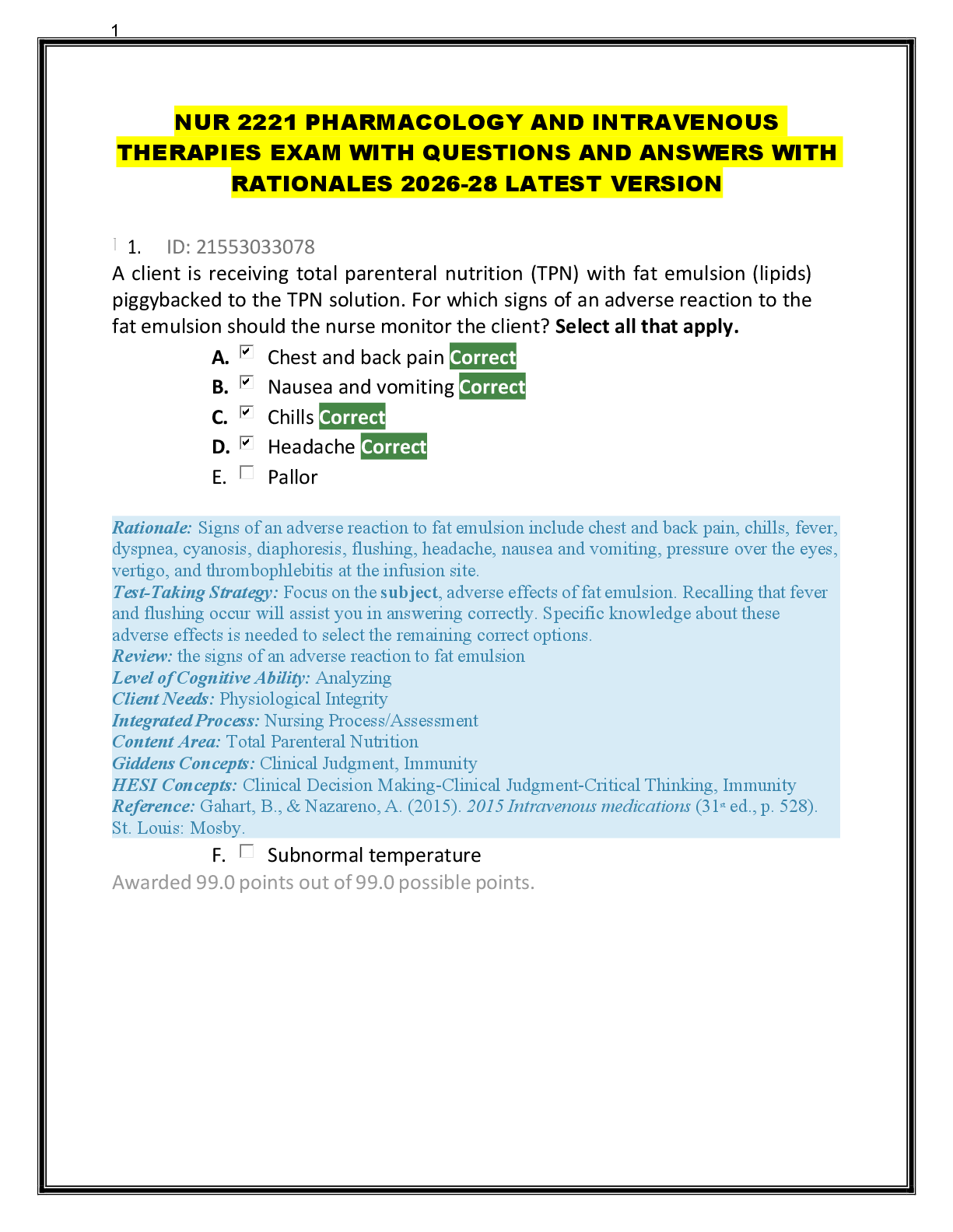
F - CORRECT ANSWER Potential energy is the energy of motion.
T - CORRECT ANSWER Potential energy is due to the composition or position of an object.
T - CORRECT AN
...
Chapter 15 Test Questions with Verified Answers
F - CORRECT ANSWER Potential energy is the energy of motion.
T - CORRECT ANSWER Potential energy is due to the composition or position of an object.
T - CORRECT ANSWER Energy exists in two basic forms, potential and kinetic energy.
T - CORRECT ANSWER The law of conservation of energy states that energy is neither created nor destroyed.
F - CORRECT ANSWER The symbol "h" is used to represent heat.
T - CORRECT ANSWER Chemical potential energy is stored in the chemical bonds of a substance.
T - CORRECT ANSWER Thermochemistry is the study of heat changes that accompany chemical reactions and phase changes.
T - CORRECT ANSWER The heat required to vaporize one mole of a liquid is called its molar enthalpy of vaporization.
F - CORRECT ANSWER The heat required to melt one mole of solid substance is called its molar enthalpy of melting.
T - CORRECT ANSWER A spontaneous process is a physical or chemical change that occurs with no outside intervention.
A - CORRECT ANSWER Calculate the energy in joules of a baked potato of 245 calories. (1 cal = 4.184 J)
A. 1.03 x 10⁶ J
B. 2.45 x 10⁵ J
C. 1.03 x 10³ J
D. 5.86 x 10⁴ J
A - CORRECT ANSWER The __________ law of thermodynamics states that energy is neither created nor destroyed.
A. first
B. second
C. third
D. fourth
A - CORRECT ANSWER The amount of energy required to raise the temperature of one gram of pure water by one degree Celsius is defined as:
A. a calorie
B. heat
C. a joule
D. a watt
C - CORRECT ANSWER The SI unit of heat and energy is the __________.
A. calorie
B. heat
C. joule
D. watt
C - CORRECT ANSWER What is the equation for calculating heat?
A. c = mq∆T
B. m = cq∆T
C. q = cm∆T
D. ∆T = cmq
D - CORRECT ANSWER How much heat is evolved from 54.0 g glucose (C₆H₁₂O₆), according to the equation for calculating heat?
C₆H₁₂O₆ + 6O₂ → 6H₂O + 6CO₂ + 2808kJ
A. 0.842 kJ
B. 8.42 kJ
C. 84.2 kJ
D. 842 kJ
B - CORRECT ANSWER For a process, the enthalpy change of a system is 1.25 x 10⁵ J and the entropy change is 300.0 J/K. Calculate the free energy change of the system at 290 K.
A. 8.7 x 10⁴ J
B. 3.8 x 10⁴ J
C. 1.2 x 10⁵ J
D. 1.2 x 10⁴ J
[Show More]