SCIENCE 101 > QUESTIONS & ANSWERS > SimBio Virtual Labs, Osmosis. SCIENCE 100 Simbio Workbook SP2020. Includes All Answers. (All)
SimBio Virtual Labs, Osmosis. SCIENCE 100 Simbio Workbook SP2020. Includes All Answers.
Document Content and Description Below
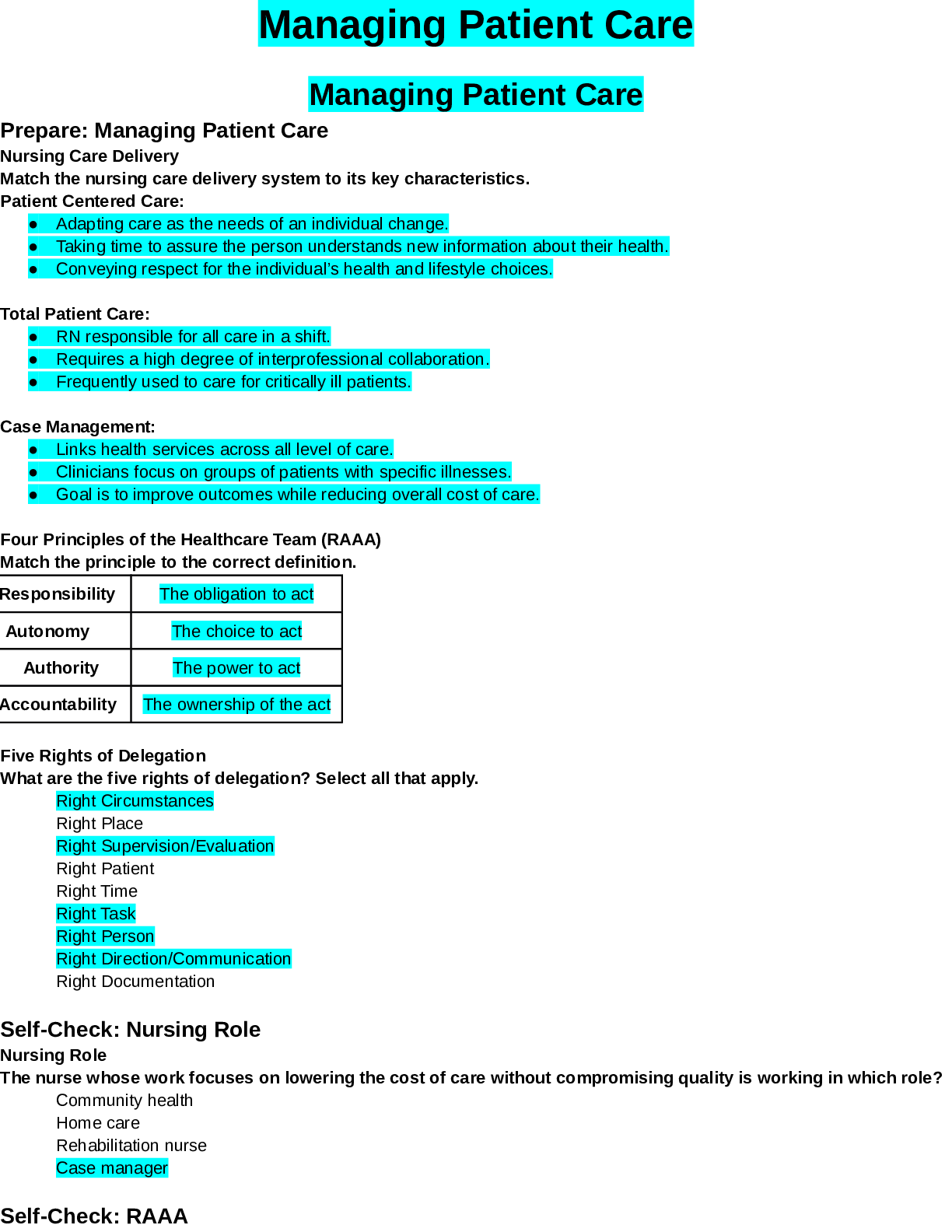
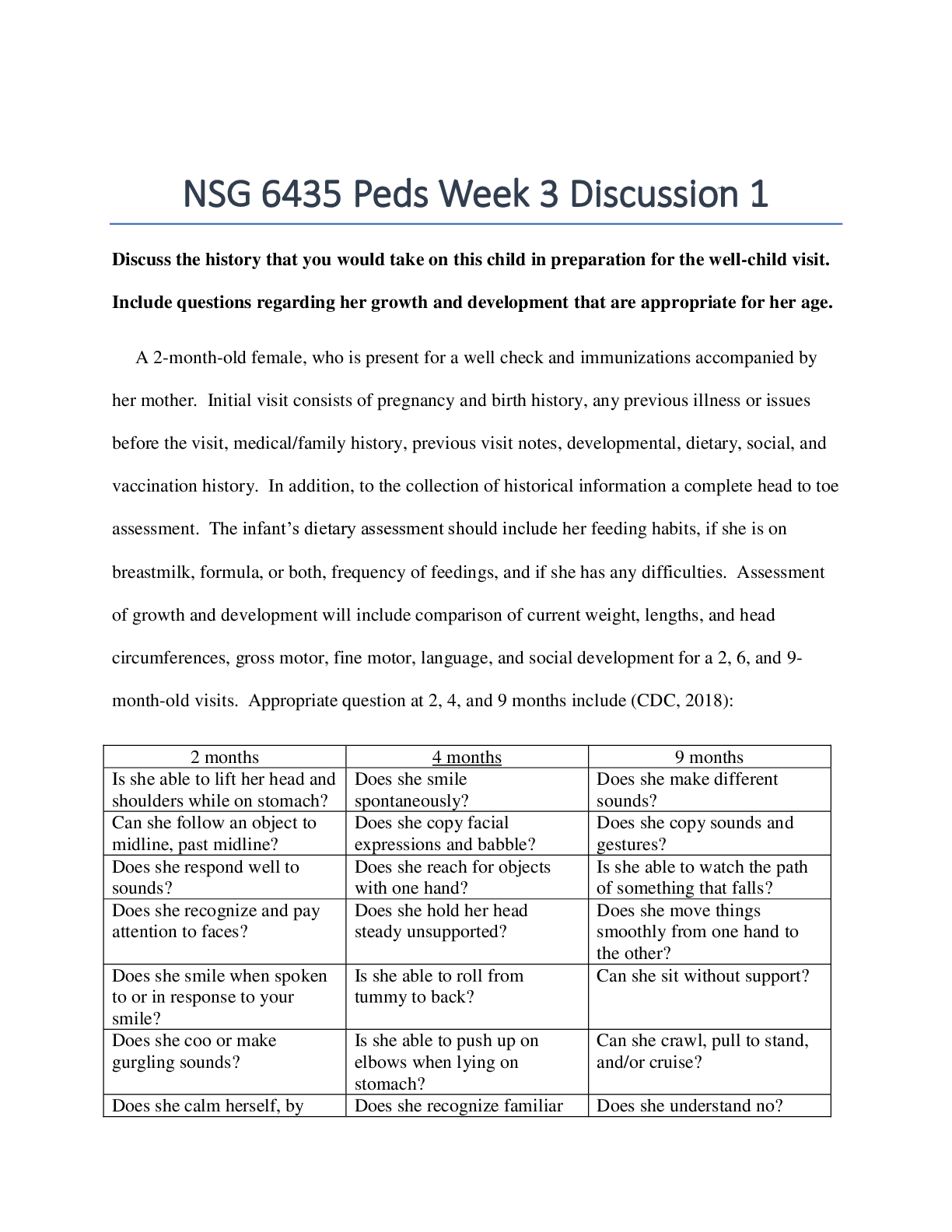
SimBio Virtual Labs® OsmoBeaker®: Osmosis NOTE TOSTUDENTS: ThisworkbookaccompaniestheSimBioVirtualLabs®Osmosislaboratory. Onlyregisteredsubscribers are authorized to use this material. Laborato... ry subscriptions may not be shared or transferred. Student’sName: Signature: Date: 4/31/2020 This and other SimBio Virtual Labs® are accessible through SimBio’s SimUText System®. This workbook, or any portion thereof, may not be reproduced or used in any manner without the express written permission from the copyright holder. Students purchasing this workbook new from authorized vendors are licensed to use the associated laboratory software; however, the accompanying software license isnon-transferable. This lab is based on work initially supported with funding from the U.S. National Science Foundation under Grant No. 0230740. . © 2019, SimBio. All Rights Reserved. 1 A WARNING FROM SIMBIO ABOUT CHEATING You should know that, among other things, we periodically tinker with the underlying models in our simulations so that the results they produce (i.e. the“right answers”) change, and we let instructors know how to recognize cheating. We hope you do not succumb to the temptation but, instead, go ahead and dive in. We’ve tried to make it a truly interesting experience and a fun way to learn. SimBio Virtual Labs®: OsmoBeaker® Osmosis Mixing Blood and Water: Osmosis andCells A patient is brought into the emergency room unconscious, with blood gushing from a wound. Among the first things you must do is get fluid into her circulatory system. If too much blood drains out, her veins could collapse. So you stick a needle into a large vein in her arm and hook up an intravenous (IV) system for delivering fluids into veins. You’re probably familiar with IVs from movies and TV shows: they are the bags hanging from a metal pole with a tube running into the patient. But what is in that bag? Is it just water, or does the water contain something? If so, does it matter what else is in the water? Later in the day, another patient comes into your clinic, a man who’s just had his 50th birthday. As standard procedure, you want to check his intestine for polyps, a possible sign of colon cancer. But the intestines are usually a bit obstructed with the remains of the last few meals. You give him pills to take with lotsof water the day before his exam.The pillsaremade of small moleculesthat areindigestible and therefore will pass right through the digestive system. This causes water to leak out into the intestine and wash it out. It’s not very pleasant for the patient, but much more pleasant for you when you do your exam. But why should some indigestible material cause water to exit his body? These are two of many of possible examples that involve osmosis, a process by which water moves across membranes in response to differences in the concentration of other molecules dissolved in the water. In this lab, you’ll take advantage of a fictional invention from a fictional bio-engineering company called Fictional Science, Incorporated. Their “Cell-O-Scope” simulator allows you to create tiny “SimCells” made out of a material that is similar to the membranes that surround animal cells. The Cell-O-Scope lets you see molecules moving around inside of the SimCells. By conducting experiments on SimCells and © 2019, SimBio. All Rights Reserved. 2 SimBio Virtual Labs® | Osmosis making observations of how the SimCells respond to different extracellular fluids, you will learn how osmosis works, and why it is an important property to consider when designing IV fluids. Concentration and Diffusion: a Brief Review Figure 1. Diffusion of Molecules Across a Permeable Membrane. A membrane that does not restrict the movement of molecules divides each box into two compartments. Comparing A to B, you can see that in each of the three examples, the number of molecules decreased on the side of the box thatstartedout more concentrated, andincreasedonthe sideofthe membrane that startedout lessconcentrated. Exercise 1: Pressure and Equilibrium [ 1 ] If you haven’t already, start SimUText® by double-clicking the program icon on your computer or by selecting it from the Start menu. [ 2 ] When the program opens, enter your Log In information and select the Osmosis lab from your Assignments window. The Cell-O-Scope Simulator (from Fictional Science, Inc.) has two monitors that let you see what happens when artificial cells (“SimCells”) are suspended in different extracellular fluids. The Cell View Monitor lets you watch the entire SimCell. The Membrane View Monitor zooms in on the SimCell membrane (the stripe down the middle of the Membrane View Monitor), letting you watch molecules move around inside the SimCell (on the left side of the membrane) and in the extracellular fluid (on the right side of the membrane). NOTE: In the real world, you could never actually see the molecules in a cell. If you could see the molecules, there would be a lot more of them! [ 3 ] The Settings Panel beneath the monitors identifies the types of molecules present, and lets you see the approximate number of each type of molecule in the cell and in the extracellular fluid. The Control Panel at the bottom of the screen runs the Cell-O-Scope Simulator. Try clicking the GO button to run the simulator. The molecules should start moving. Notice that the movement of any individual molecule is random. Then click the STOP button. [ 4 ] Make sure that the simulator is STOPPED, then push the cell membrane by clicking on the membrane and dragging it to the left. Watch the SimCell in the Cell View Monitor change shape as the membrane is compressed. Next, drag the membrane to the right to stretch the membrane. The relative areas you see in the Membrane View are proportional to the relative volumes of the SimCell and extracellular fluid. You can assume that when the membrane position is in the middle (at 20 on the Membrane Position scale), that the SimCell occupies half of the volume of the Cell-O-Scope. [ 4.1 ] What happens to the membrane (visible in the Membrane View Monitor), when you try pushing it all the way to the right? (If you’re not sure what happened, click the RESET button in the Control Panel and try again.) [ 4.2 ] What happens to the SimCell (visible in the Cell View Monitor) when you try pushing the membrane all the way to the right? [ 5 ] Click the RESET button in the Control Panel. Then click the GO button. The molecules on either [ 5.1 ] If you increase the number of water molecules in the extracellular fluid, will the membrane move to the right or the left in the Membrane View? Explain your reasoning in terms of pressure. [ 7.1 ] Was your prediction in Question 5.1 correct? Whathappened? [ 9.1 ] How can you tell when the pressures have equalized? SimBio Virtual Labs® | Osmosis © 2019, SimBio. All Rights Reserved. 5 [ 9.2 ] What is the approximate “equilibrium” position of the membrane? 19.7 [ 9.3 ] If you continue to run the simulator after the system has reached equilibrium, why does the membrane continue to jiggle back and forth? [ 10.1 ] Was the membrane at a stable equilibrium before you pushed it? How could you tell? [ 11.1 ] Based on how the molecules moved at different temperatures, which of the below choices (a, b, or both) do you think is correct? [Note: you’ll experiment with this in the next step; for now simply make a prediction.] a. at higher temperatures, the equilibrium position of the membrane should shift to the right. b. at higher temperatures, the system should equilibrate faster. [ 11.2 ] Explain your choice(s) [ 12.1 ] Describe the experiment(s) you tried and what you figured out. Does temperature influence the equilibrium position of the membrane? Does temperature influence how long it takes for the system to equilibrate? Explain your results in terms of pressure. Exercise 2: Osmotic Pressure So far, your membrane has been impermeable (nothing can pass through it), and the SimCell and extracellular fluid have only contained water. Real cells contain water and lots of other types of molecules dissolved in the water. Water is referred to as the solvent in cells, because most of the molecules in real cells are water molecules. The molecules dissolved in the water are referred to as solutes. Real cells also have channels that allow water to pass through their membranes. Osmosis is the diffusion of water across a semi-permeable membrane—a membrane that is permeable to some molecules (such as water) but not others (such as dextrose). If water molecules diffuse across a membrane into a cell, the pressure of the added molecules will cause the membrane to stretch and the cell to grow. [ 1 ] Select Osmotic Pressure 1 from the SELECT AN EXERCISE button in the upper left-hand corner of the screen. [ 2 ] The new SimCell contains 200 water molecules and 50 dextrose molecules. The extracellular fluid contains 250 moles of water molecules, so the total number of molecules is the same on either side of the membrane (250). Click the GO button to confirm that the pressure on either side of the membrane is the same. [ 2.1 ] How can you tell that there is no major difference in pressure on either side of the membrane? [ 3.1 ] Will the membrane move to the right, left, or stay in the same position? Explain your reasoning. I think that the membrane will move to the right because the dextrose molecules might need more space. SimBio Virtual Labs® | Osmosis © 2019, SimBio. All Rights Reserved. 8 [ 4 ] Add water channels by checking the box next to the water molecule icon in the Membrane Permeability [ 4.1 ] Were you correct? What happened? Osmotic pressure is the pressure generated by osmosis. Technically, itis the minimum pressure that must be applied to a solution to prevent water from flowing across a semipermeable membrane into the solution. [ 5.1 ] Do you predict that the SimCell will expand or shrink? Why? SimBio Virtual Labs® | Osmosis © 2019, SimBio. All Rights Reserved. 9 [ 6.1 ] Were you correct? What happened? Exercise 3: Osmosis and Concentration [ 1 ] Select Osmosis and Concentration 1 from the SELECT AN EXERCISE button in the upper [ 2.1 ] What is the solute concentration (%) inside the SimCell? Show how you set up your calculations in the space below. [ 2.2 ] What is the concentration (%) of water molecules inside the SimCell? Show how you figured this out. [ 3.1 ] Do you predict that the membrane will shift to the left, the right, or neither? Explain your reasoning. [ 4.1 ] Was your prediction in Question 3.1 correct? [ 4.2 ] Complete the table below by filling in the equilibrium numbers of molecules. You can read these from the Current columns for the Cell and the Extracellular Fluid. [Note: there is a calculator tool at the bottom of your screen.] CELL EXTRACELLULAR FLUID Water 275 150 Dextrose 50 25 Total* 325 175 * the two totals should add up to 500! [ 4.3 ] Using the numbers in the table, circle the closest concentrations (%) for the two sets of choices given below. [Note: the choices won’t exactly match your ratios; just pick the ones that are closest]. Approximate concentration of water inside cell: 75% 85% 95% Approximate concentration of water outside cell: 75% 85% 95% [ 4.4 ] In the space below, explain why the concentration of water should be nearly the same inside the cell and in the extracellular fluid, at equilibrium. Concentration of water should be nearly the same inside the cell and in the extracellular fluid because water concentrations diffuse throughout the cell membrane. 12 [ 4.5 ] Was the solution you used hypertonic, hypotonic, or isotonic relative to the cell? Explain your choice. [ 5.1 ] The SimCell cell would have expanded less—or shrunk—if the initial concentration of dextrose molecules in the extracellular fluid had been: circle one: lower / higher [ 5.2 ] Explain your choice. compared to the inside. [ 5.3 ] What should the initial number of dextrose and water molecules in your IV fluid be for the extracellular fluid to be isotonic to the cell? (!) Explain how you obtained your answer. [ 6.1 ] In the space below, note the starting position of the membrane so you can tell whether the cell changes size. 20 [ 7.1 ] Did the position of the membrane remain about the same? Yes / No [ 7.2 ] Did you succeed in making an isotonic solution? Yes / No SimBio Virtual Labs® | Osmosis © 2019, SimBio. All Rights Reserved. 13 [ 7.3 ] If you did NOT succeed in making an isotonic solution (i.e., the membrane shifted to a new equilibrium), keep trying until you figure it out, and describe what you discovered in the space below. [ 8.1 ] Calculate the initial concentration of glucose and of all solutes combined in the SimCell. (The calculation for dextrose is shown as an example.) Show your calculations! Dextrose: 30 / (200 + 30 + 20) = 30 / 250 = 0.12. 0.12 x 100 = 12% Glucose: 20/(200+30+20)=20/250= 0.08 *100= 8% Total solute: 50/250= 0.2*100= 20% [ 8.2 ] Based on the concentration of solute, what is the concentration of water in the SimCell? 1-0.2=0.8*100=80% [ 9.1 ] How many dextrose and how many water molecules are needed in the extracellular fluid so the fluid will be isotonic with the cell? [ [ 10.1 ] Did the equilibrium cell position shift, indicating the cell expanded or contracted? Yes / No (If it did, try again and record the values you try below.) [ 11 ] Click the TEST YOUR UNDERSTANDING button in the bottom right corner of the screen. You can 14 Exercise 4: Red Blood Cell [ 1 ] Select Red Blood Cell from the SELECT AN EXERCISE button in the upper left-hand corner of the screen. [ 1.1 ] How many molecules are in the artificial RBC? 180 How many molecules are in the extracellular fluid? 360 [ 1.2 ] [ 1.3 ] What is the concentration (%) of solute in the RBC (i.e., what is the concentration of all solute molecules combined?) As before, show your calculations. You might find it [ 1.4 ] What is the concentration (%) of solute in the extracellular fluid? Show your calculations. [ 2.1 ] Using your calculations above, do you predict that the RBC will shrink, expand, or stay the same size if the membrane is made permeable to water? [ 2.2 ] Circle the correct words in the brackets below to accurately complete the sentence. Because the IV fluid is [ hypertonic / hypotonic / isotonic ] to the RBC, the RBC will [ grow / shrink / stay the same size ]. [ 3 ] Click the Membrane Permeability box for Water and run the simulator to test your prediction. [ 3.1 ] Was your prediction in Question 2.1 correct? [ 4.1 ] Use the space below to show your calculations for how much dextrose you need to make an isotonic IV fluid for this cell so it doesn’t change size. [ 4.2 ] Check your new IV fluid by running the simulator. After you have found a fluid that works, record its composition in the space below. [ 4.3 ] You want to save the next doctor who comes along some time figuring all this out. In the space below, write a short list of instructions on how to compose IV fluids based on your work in this lab. [ 5 ] Click the TEST YOUR UNDERSTANDING button in the bottom right corner of the screen. You can click as many answers as you like to verify that you understand. 16 Mystery Cell (Optional) [ 1 ] Select Mystery Cell from the SELECT AN EXERCISE button in the upper left-hand corner of the screen. This SimCell is mysterious, because its contents are not visible. [ 4.1 ] First, what is the approximate concentration of solute molecules in the mystery RBC at equilibrium? Explain how you determined this value. [Hint: if all solute molecules [ 5.1 ] What is the concentration (%) of hemoglobin in the cell? Show or describe how you figured this out, attaching additional sheets if necessary. Graded Questions [ 1 ] Use the SELECT AN EXERCISE button in the upper left-hand corner of the screen to launch “Graded Questions”. Notes and Comments [Show More]
Last updated: 2 years ago
Preview 1 out of 20 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$12.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Mar 09, 2021
Number of pages
20
Written in
Additional information
This document has been written for:
Uploaded
Mar 09, 2021
Downloads
0
Views
163