.png)
Pediatric Quick Notes | Download To Score An A
$ 10

ATI COMPREHENSIVE 2022 NEW UPDATED AND 100% COMPLETE STUDY GUIDE SOLUTION
$ 15

Read the speech delivered by Christopher Reeve, and accomplish the following activities afterwards. Analyze the text which uses proposition and support patterns.
$ 9.5

Supply Chain Management at Walmart Case study
$ 11

Fresenius ~ Water Treatment Study Guide COMPLETED
$ 10

Auditing The Art and Science of Assurance Engagements, 15th Canadian Edition By Alvin Arens, Randal Elder, Mark Beasley, Chris | [eBook] [PDF]
$ 29
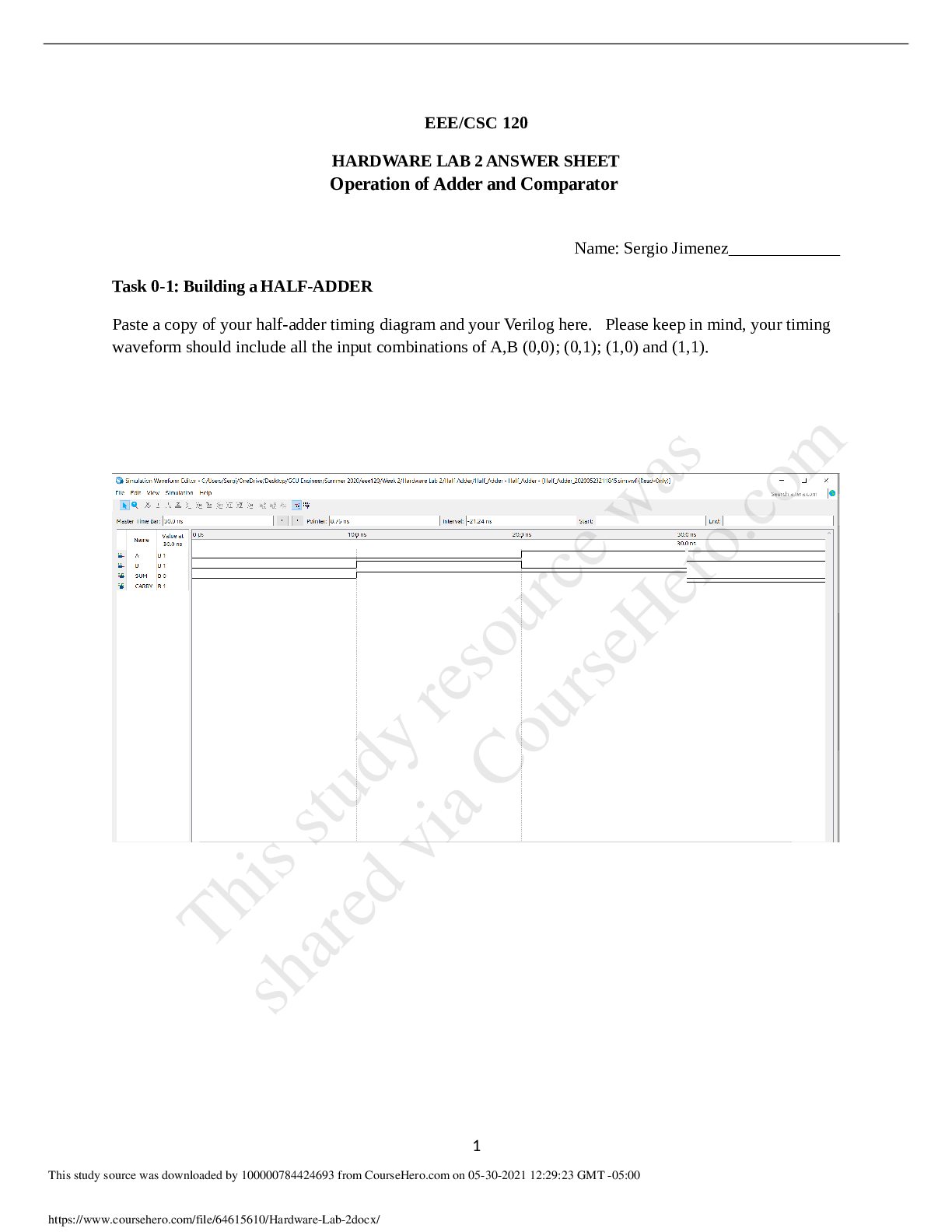
EEE/CSC 120 HARDWARE LAB 2 ANSWER SHEET Chandler-Gilbert Community CollegeCSC 120Hardware Lab 2.ALL ANSWERS CORRECT
$ 9

AQA GCSE RELIGIOUS STUDIES A 8062/2A Paper 2A: Thematic Studies Question Paper November 2021
$ 8

Solution Manual and Cases for Nursing Diagnosis Handbook An Evidence-Based Guide to Planning Care 12th Edition
$ 15
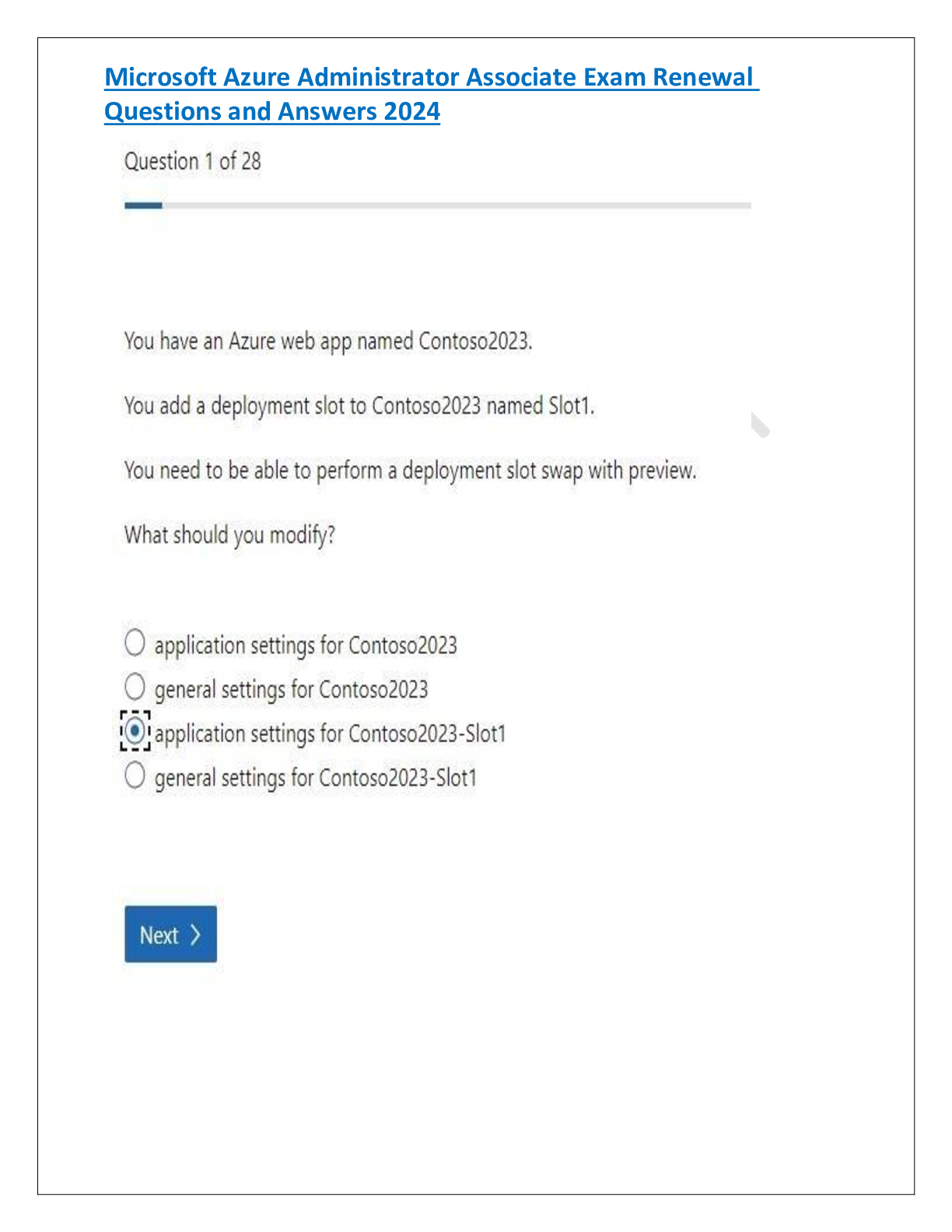
Microsoft Azure Administrator Associate Exam Renewal Questions and Answers 2024
$ 33

APPLIED DECISION METHODS GBA 334-Midterm-Saint Leo University-2019-A+ Graded 100%
$ 9

The City College of New York, CUNY - ME 472Report #5. Mechanical Systems Design Boundary Lubricated Bearing
$ 5

PRACTICE EXAM QUESTIONS AND ANSWERAS SOLUTION NCLEX RN 13
$ 15.5
.png)
ITM750 - final exam review_ questions and answers_ chapters 1-13
$ 15

PACO 501 Exam 2; Latest 2019/20 Complete solution, Attempt score; 48 Out of 50 points.
$ 7

TEST BANK FOR Analytics, Data Science, & Artificial Intelligence Systems for Decision Support, 11 Edition. Ramesh Sharda, Dursun Delen
$ 18

Solutions Manual For Applied Calculus 3rd Edition By Hughes-Hallett Thrash
$ 30
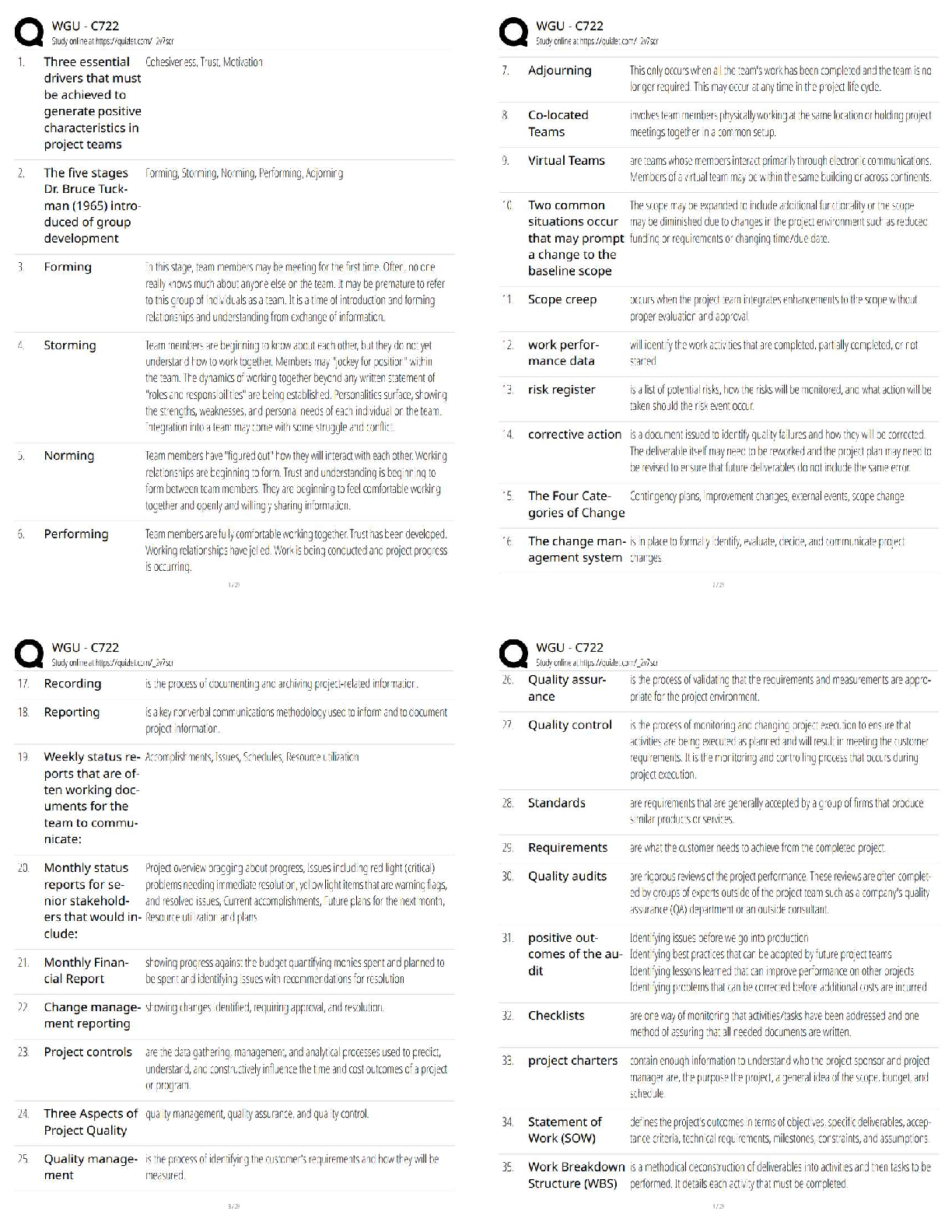
WGU C722 / Project Management / Score 100% / New Version / 2025 Update / Study Guide & Test Bank
$ 10

WGU C182 EXAM WITH COMPLETE SOLUTION
$ 6.5

AQA GCSE GEOGRAPHY 80351 Paper 1 Living With The Physical Environment
$ 10
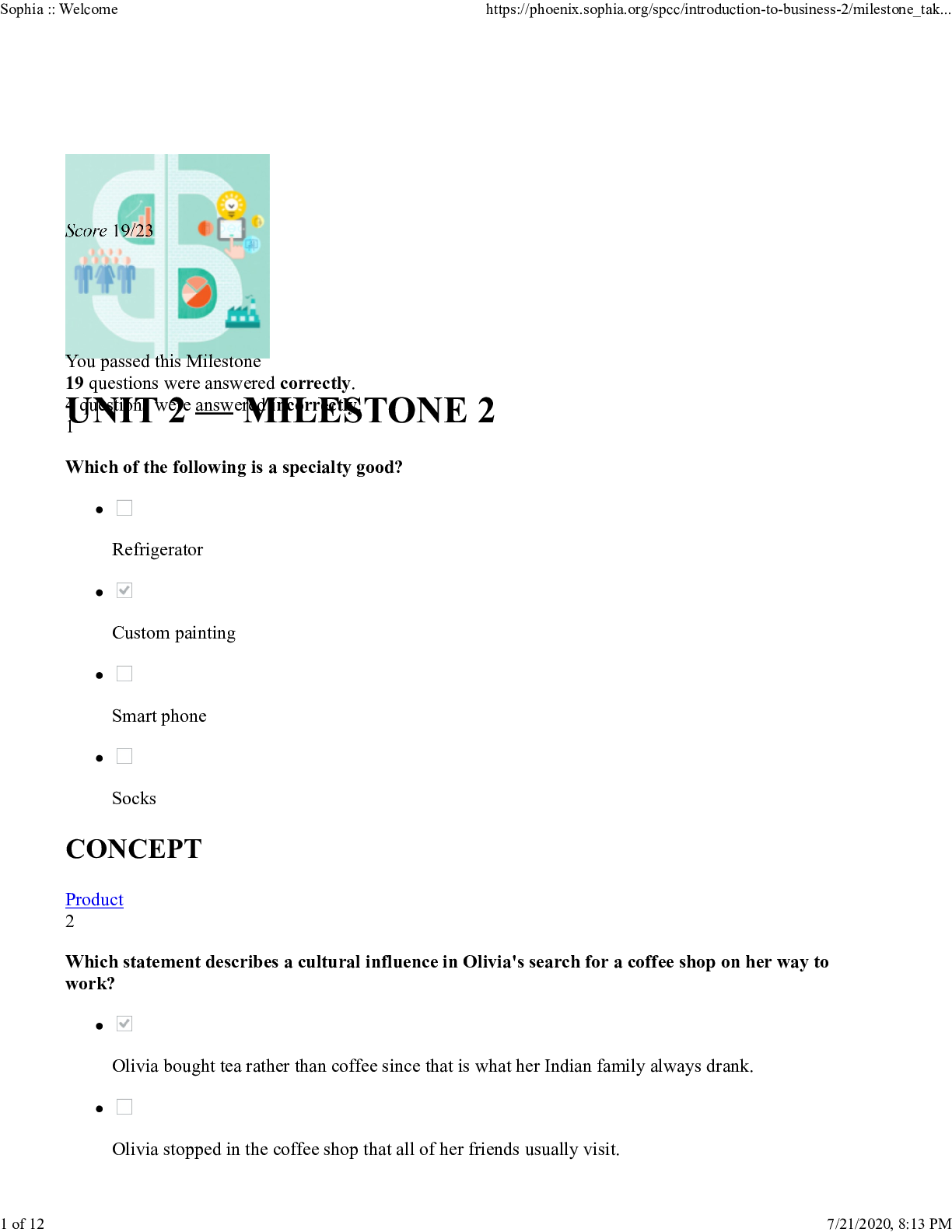
Introduction to Business Milestone 2 Test.pdf. COMPLETE QUESTIONS AND ANSWERS