Chemistry > QUESTIONS & ANSWERS > Questions and Answers > Florida Atlantic University - CHM 2211CHM2211_Practice Final-1. All answers (All)
Questions and Answers > Florida Atlantic University - CHM 2211CHM2211_Practice Final-1. All answers highligted.
Document Content and Description Below
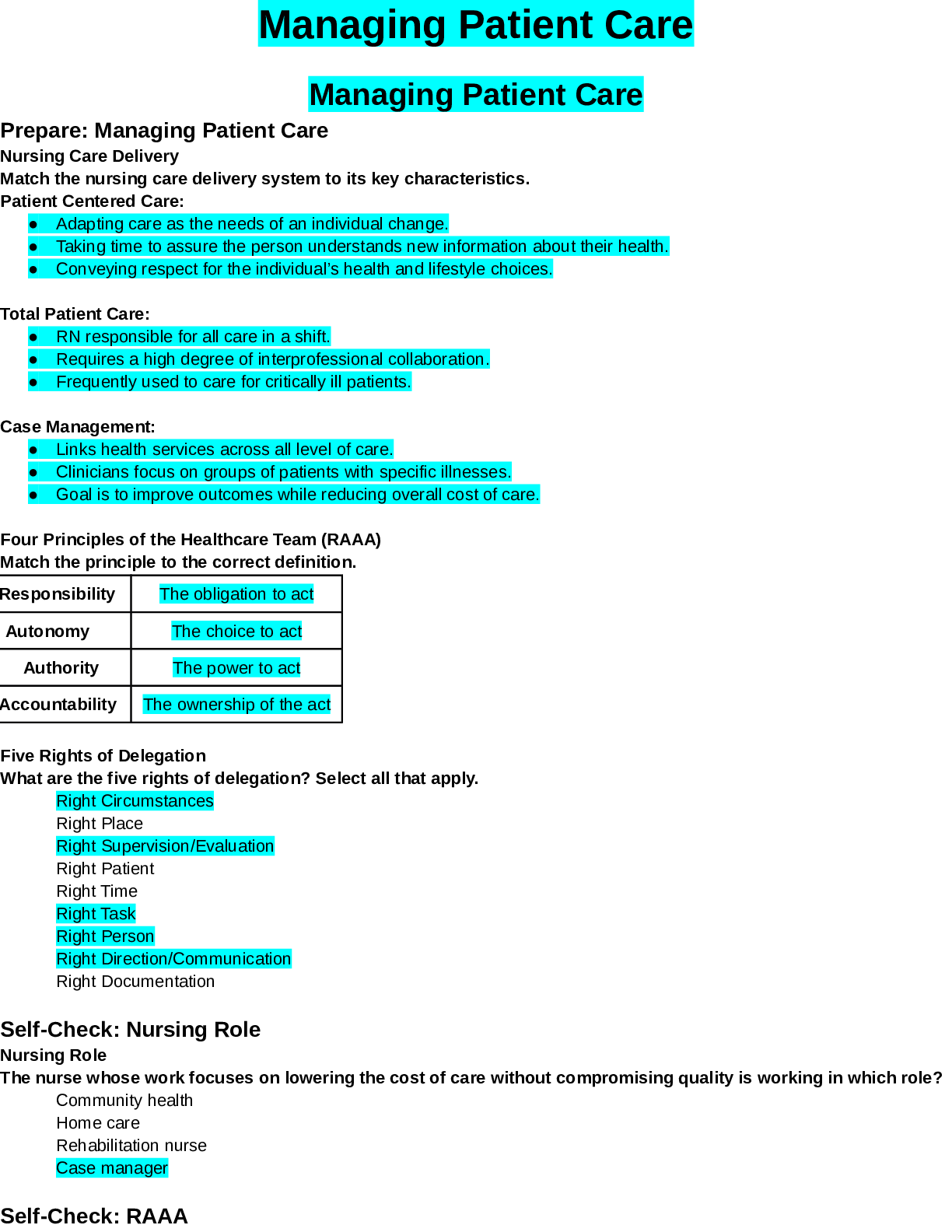
1. Which of these compounds could not be formed by nucleophilic attack by an appropriate reagent on acetyl chloride? A) B) C) D) E) 2. The product of the following reaction would be: A) B) ... C) D) E) Page 13. Predict the major organic product of the reaction sequence, A) I B) II C) III D) IV E) V 4. The product of the following reaction is: A) I B) II C) III D) IV E) V Page 25. A compound with the molecular formula C5H10O2 gave the following 1H NMR spectrum: triplet, 0.90 multiplet, 1.60 singlet, 1.95 triplet, 3.95 The IR spectrum showed a strong absorption band near 1740 cm-1. The most likely structure for the compound is: A) B) C) D) E) 6. Intramolecular dehydration to form a cyclic monoester is most likely to occur when which of the following is heated with acid? A) CH3CH2CH2CHOHCO2H B) CH3CH2CHOHCH2CO2H C) CH3CH2CH2CH2CO2H D) CH3CHOHCH2CH2CO2H E) HO2CCH2CH2CO2H Page 3Consider the reaction sequence below to answer the following questions: 7. Compound X, diethyl propanedioate, is more commonly known as ___________. a. ethyl acetoacetate b. acetoacetic ester c. oxalic ester d. malonic ester 8. What would be the major product of the following reaction? A) B) C) D) E) Page 4 EtO OEt O O 1. NaOEt, EtOH 2. O Br O CO2Et CO2Et NaOEt, EtOH CompoundZ CompoundX CompoundY9. The best synthesis of would be: A) B) C) D) E) All of these are equally good. 10. Which reagent would you use to carry out the following transformation? tert-butylbenzene p-tert-butylbenzenesulfonic acid +o -tert-butylbenzenesulfonic acid A) HNO3/H2SO4 B) tert-C4H9Cl/AlCl3 C) H2SO3/peroxides D) SO3/H2SO4 E) SO2/H2SO3 Page 511. The major product(s), B, of the following reaction, would be: A) I B) II C) III D) Equal amounts of I and II E) Equal amounts of I, II and III 12. Why would 1,3-cyclohexadiene undergo dehydrogenation readily? A) It is easily reduced. B) Hydrogen is a small molecule. C) 1,3-Cyclohexadiene has no resonance energy. D) It would gain considerable stability by becoming benzene. E) It would not undergo dehydrogenation. 13. This compound is most precisely named: A) sec-Hexylbenzene B) 2-Phenylhexane C) (R)-2-Phenylhexane D) (S)-2-Phenylhexane E) Butylmethylphenylmethane Page 614. Which dibromobenzene can, in theory, yield three mononitro derivatives? A) o-Dibromobenzene B) m-Dibromobenzene C) p-Dibromobenzene D) All of these E) None of these 15. In which case is the indicated unshared pair of electrons NOT a contributor to the aromatic system? A) I B) II C) III D) IV E) None of these 16. On the basis of molecular orbital theory and Huckel's rule, which molecules and/or ions should be aromatic? A) I and V B) III and VIII C) IV, VII and IX D) IV, VI, VII and IX E) All of the structures, I-IX Page 717. Which of the following structures would be aromatic? A) I B) II C) III D) IV E) V 18. Which annulene would you NOT expect to be aromatic? A) [6]-Annulene B) [14]-Annulene C) [16]-Annulene D) [18]-Annulene E) [22]-Annulene 19. Which of the following would have the longest carbon-carbon bond? A) I Page 820. We now know that the two Kekule structures for benzene are related in the following way: A) They are each equally correct as a structure for benzene. B) Benzene is sometimes one structure and sometimes the other. C) The two structures are in a state of rapid equilibrium. D) Neither of the two structures adequately describes benzene; benzene is a resonance hybrid of the two. E) None of the above 21. Which alkene would you expect to be most stable? Page 922. Which free radical would be most stable? 23. Which of these dienes is the most reactive in the Diels-Alder reaction? A) 1,3-Butadiene B) 1,4-Pentadiene C) Cyclopentadiene D) 1,2-Butadiene E) 1,4-Cyclohexadiene 24. Which of the following dienes is a cumulated diene? A) CH2=CHCH2CH2CH=CH2 B) CH2=CHCH=CHCH2CH3 C) CH3CH=C=CHCH2CH3 D) CH3CH=CHCH=CHCH3 E) CH3CH=CHCH2CH=CH2 25. This substituent deactivates the benzene ring towards electrophilic substitution but directs the incoming group chiefly to the ortho and para positions. A) -NO2 B) -F C) -CF3 D) -NHCOCH3 E) -OCH2CH3 Page 1026. Select the structure of the major product formed in the following reaction. CH3CH O CH2 HA H218O ? A) CH3CH2CH218OH B) CH3CHCH3 18OH C) CH3CHCH2OH 18OH D) CH3CH OH CH2 18OH E) CH3CHCH218OH 18OH 27. From the standpoint of reactivity, which is the poorest choice of dienophile to react with in a Diels-Alder reaction. A) B) C) D) CH3O2C-CC-CO2CH3 E) Page 1128. Which reagent would convert 1,3-pentadiene into the alcohol shown below? A) KMnO4/-OH B) OsO4 C) H2O2, then H3O+ D) Cl2/H2O E) H3O+ 29. Select the most energetically favorable UV transition for 1,3-butadiene. A) n * B) n * C) 2 3* D) * E) 1 4* 30. Which is a correct IUPAC name for CH3OCH2CH2OCH3? A) 1,4-Dioxane B) Ethylene glycol dimethyl ether C) 1,4-Dioxapentalene D) 1,2-Dimethoxyethane E) 1,2-Diethoxymethane 31. is properly named: A) cis-1,2-Cyclopentanediol B) meso-1,2-Cyclopentanediol C) (1R,2R)-1,2-Cyclopentanediol D) (1R,2S)-1,2-Cyclopentanediol E) (1S,2S)-1,2-Cyclopentanediol Page 1232. Which is the best way to prepare isopropyl methyl ether via the Williamson method? A) CH3OH + (CH3)2CHOH + H2SO4, 140C B) CH3OH + (CH3)2CHCH2OH + H2SO4, 140C C) CH3ONa + (CH3)2CHBr D) CH3I + (CH3)2CHONa E) CH3I + (CH3)2CHCH2ONa 33. Which would be the best method for converting A into B? A) H3O+, heat B) BH3:THF; then H2O2, OHC) concd. H2SO4; then H2O, heat D) Hg(OAc)2/THF-H2O; then NaBH4,OHE) HBr; then NaOH/H2O 34. Which compound would have the lowest solubility in water? A) Diethyl ether B) Methyl propyl ether C) 1-Butanol D) 2-Butanol E) Pentane Page 1335. Which alcohol would undergo acid-catalyzed dehydration most rapidly? 36. What is the predominant product from the reaction of 2-hexanol with H2CrO4? A) CH3CO2H B) CH3(CH2)3CO2H C) D) CH3(CH2)4CO2H E) A) and B) Page 1437. How could the following synthesis be accomplished? A) (1) SOCl2, (2) Mg, ether, (3) then H3O+ B) (1) SOCl2, (2) Li, ether, (3) (CH3CH2)2CuLi, (4) KMnO4, OHC) 1) PBr3, (2) Mg, ether, (3) then H3O+ (4) PCC,CH2Cl2 D) More than one of the above E) None of the above 38. Which of the following statements is true about the anion formed from the reaction of diethyl malonate with sodium ethoxide? A) It can react with an , -unsaturated ester by conjugate addition. B) It can condense with aldehydes and ketones. C) It can be alkylated with an alkyl halide. D) It is resonance stabilized. E) All of the above statements are true. Page 1539. Consider the synthesis above. What is compound X? 40. Consider the synthesis above. What is compound Y? Page 1641. Consider the synthesis above. What is compound Z? 42. Fischer esterification is an example of: A) nucleophilic acyl addition B) nucleophilic acyl elimination C) nucleophilic acyl substitution D) nucleophilic acyl rearrangement Page 1743. Which of these amines is/are used with aldehydes and ketones to form enamines? A) I B) II C) III D) IV E) Both I and II 44. Which base is employed in the alkylation of ethyl pentanoate with methyl iodide? A) Sodium methoxide B) Sodium ethoxide C) Sodium hydride D) Potassium tert-butoxide E) Lithium diisopropylamide 45. How many 1H NMR signals would the following compound give? A. 1. PhMgBr, ether B. 1. PhCH2MgBr, ether 2. H3O+ 2. H3O+ C. (C6H5)3P=CHC6H5, THF D. Li(C6H5)2Cu, ether Page 18 ORank the following groups of compounds from most acidic (1) to least acidic (4). Place the number corresponding to the compound's relative rank in the blank below the structure. 49. How many equivalent resonance structures can be written for the cyclopentadienyl anion? 50. The 1H NMR signal for which of the indicated protons is the most shielded? Page 19 CN51. Which structure represents an ester enolate? E) Both B) and C) 52. Which reaction is an oxidation? E) All of these For the molecules below with the most acidic hydrogens are underlined. 53. Rank the molecules above in order of increasing acidity (least acidic to most acidic). OHConsider the reaction below to answer the following questions. 54. The weakest acid in the reaction is: D 55. The strongest base in the reaction is: B 56. The enolate ion in the reaction is: C 57. Which of the following represent tautomers? D) All of these E) None of these Page 2158. Which reaction is a haloform reaction? 59. Which of the following statements best describes the base peak in a mass spectrum? A) The peak from the most stable radical. B) The peak from the species that has the isotope with the highest atomic number. C) The peak of highest intensity. D) The peak from the molecule minus an electron. 60. When an external magnetic field is applied, what happens to the protons in a sample? A) All protons align with the field. B) All protons align opposite to the field. C) Some protons align with the field and some align opposite to it. D) All protons assume a random orientation 61. Which statement about 1H NMR is true? A) The integration indicates the relative number of each type of hydrogens. B) Chemical shift is a measure of the reactivity of the compound for which the spectrum is obtained. C) The chemical shift of a proton is proportional to the number of hydrogens on adjacent carbons. D) Integration depends on the size of the applied magnetic field. Page 2262. Vicinal coupling is: A) coupling between 1H nuclei attached to adjacent C atoms. B) coupling between 1H nuclei in an alkene. C) coupling between 1H nuclei attached to the same C atom. D) coupling between 1H nuclei in an alkane. Consider the reaction below to answer the following questions: 63. The substance formed on addition of water to an aldehyde or ketone is called a hydrate or a/an: A) vicinal diol B) geminal diol C) acetal D) ketal 64. Many nucleophilic addition reactions of aldehydes and ketones are catalyzed by acid or base. Bases catalyze hydration by: a. making the carbonyl group more electrophilic b. shifting the equilibrium of the reaction c. making the carbonyl group less electrophilic d. converting the water to hydroxide ion, a much better nucleophile Page 23 O HO OH H2O base catalyst + [Show More]
Last updated: 3 years ago
Preview 1 out of 23 pages
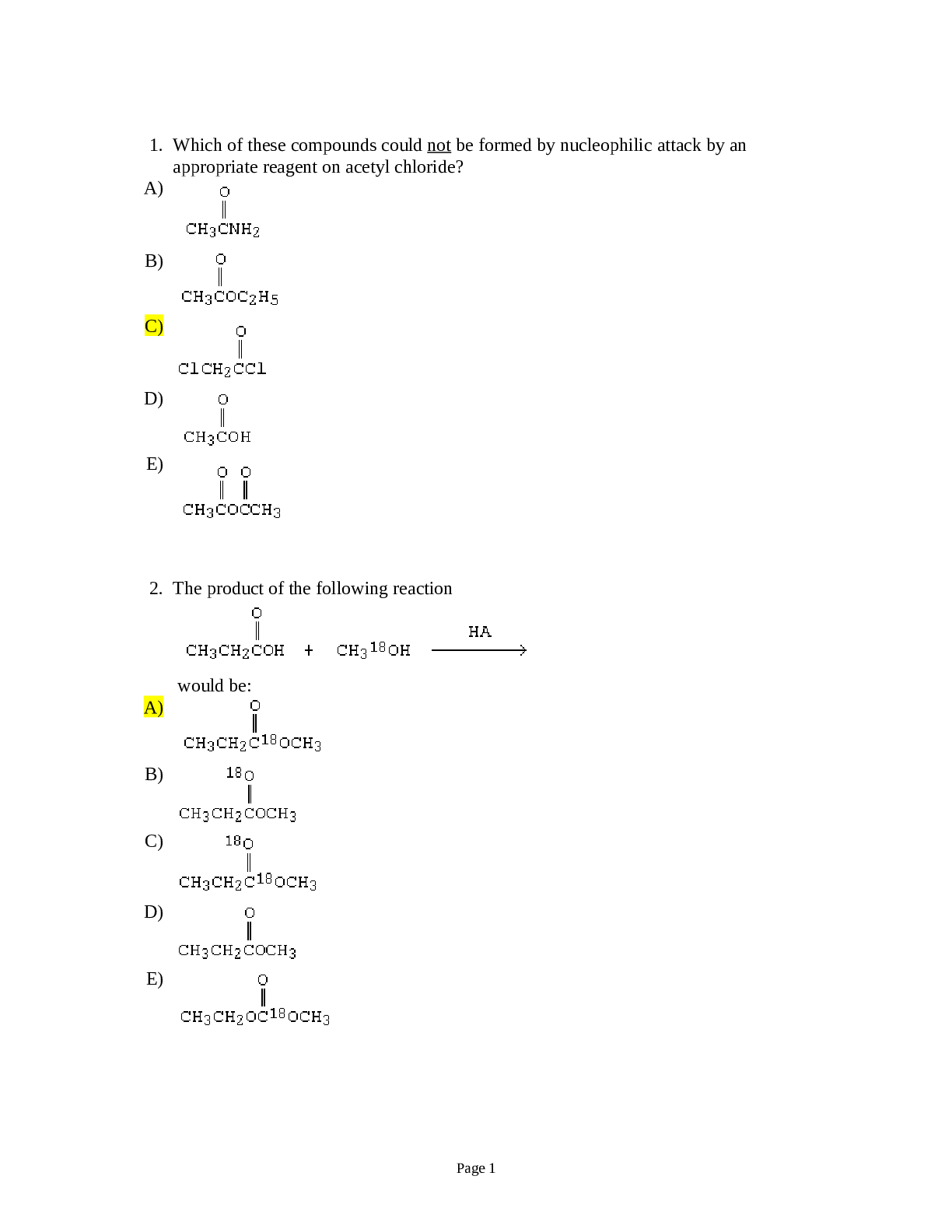
Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$12.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Apr 07, 2021
Number of pages
23
Written in
All
Additional information
This document has been written for:
Uploaded
Apr 07, 2021
Downloads
0
Views
106