Chemistry > QUESTIONS & ANSWERS > Package Title: Solomons organic chemistry Test Bank Course Title: Solomons 12the Edition. Chapter Nu (All)
Package Title: Solomons organic chemistry Test Bank Course Title: Solomons 12the Edition. Chapter Number: 17
Document Content and Description Below
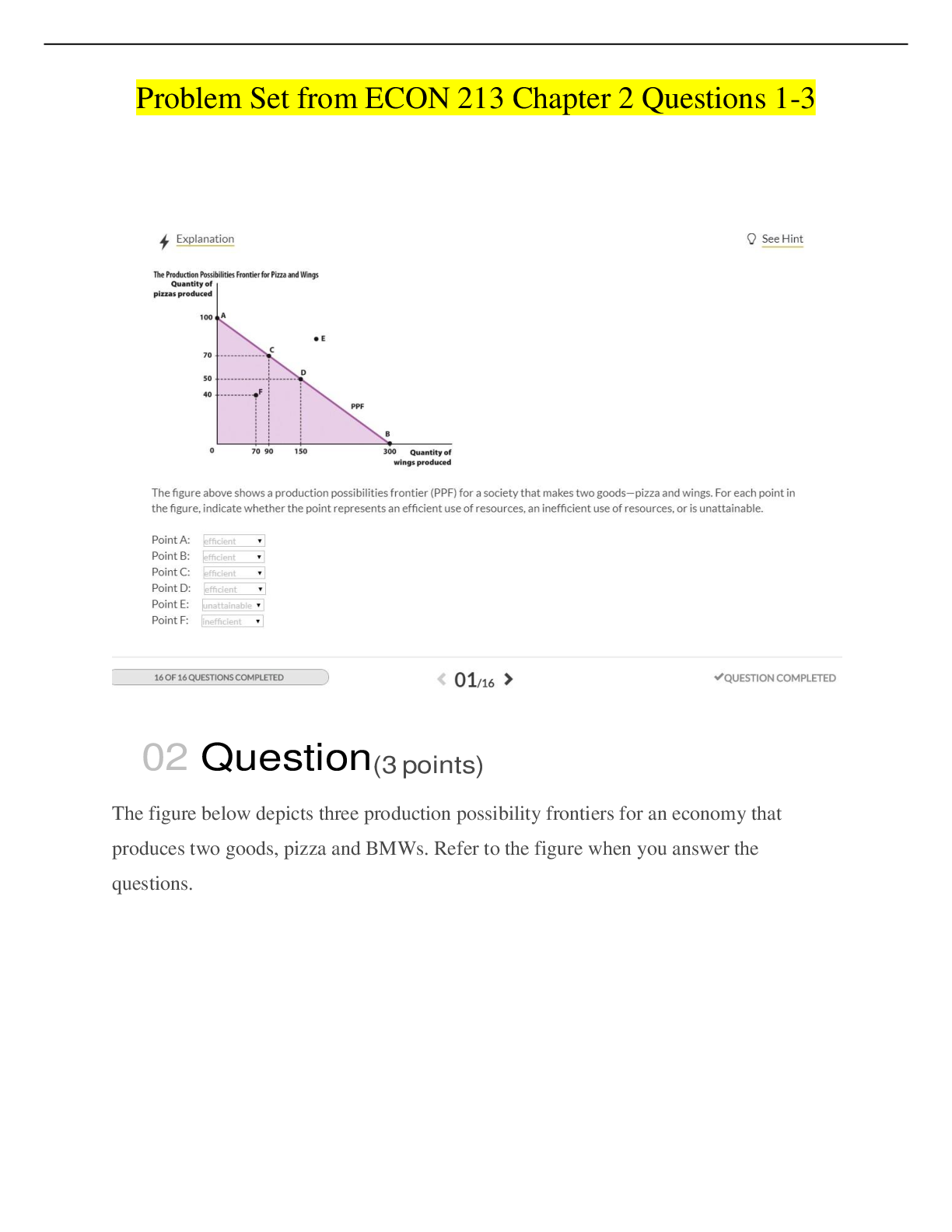
Which of the following is the best name for the following compound? O O a) Isobutyl ethanoate b) Ethyl isopropanoate c) 3-methylbutyl ethanoate d) Ethoxy isobutyl ketone e) Ethyl 3- ... methylbutanoate Answer: E Topic: Nomenclature Section: 17.2 Difficulty Level: Medium 2) The correct structure for ethyl 3-methylbutanoate is: O O O O O O I II III O O O O IV V a) I b) II c) III d) IV e) V Answer: D Topic: Nomenclature Section: 17.2 Difficulty Level: Medium 1 Which of the following is the best name for the following compound? O O a) Isobutyl ethanoate b) Ethyl isopropanoate c) 3-methylbutyl ethanoate d) Ethoxy isobutyl ketone e) Ethyl 3-methylbutanoate Answer: E Topic: Nomenclature Section: 17.2 Difficulty Level: Medium 2) The correct structure for ethyl 3-methylbutanoate is: O O O O O O I II III O O O O IV V a) I b) II c) III d) IV e) V Answer: D Topic: Nomenclature Section: 17.2 Difficulty Level: Medium 1 3) The correct structure for bicyclo[1.1.1]pentane-2-carboxylic acid is: a) I b) II c) III d) IV e) V Answer: D Topic: Nomenclature Section: 17.2 Difficulty Level: Medium 2 4) The correct structure for bicyclo[2.2.2]octane-2-carboxylic acid is: a) I b) II c) III d) IV e) V Answer: B Topic: Nomenclature Section: 17.2 Difficulty Level: Medium 3 5) The correct structure for bicyclo[1.1.0]butane-2-carboxylic acid is: a) I b) II c) III d) IV e) V Answer: E Topic: Nomenclature Section: 17.2 Difficulty Level: Medium 4 6) The correct structure for methyl bicyclo[1.1.1]pentane-2-carboxylate is: a) I b) II c) III d) IV e) V Answer: D Topic: Nomenclature Section: 17.2 Difficulty Level: Medium 5 7) The correct structure for methyl bicyclo[2.2.2]octane-2-carboxylate is: a) I b) II c) III d) IV e) V Answer: B Topic: Nomenclature Section: 17.2 Difficulty Level: Medium 6 8) The correct structure for methyl bicyclo[1.1.0]butane-2-carboxylate is: a) I b) II c) III d) IV e) V Answer: E Topic: Nomenclature Section: 17.2 Difficulty Level: Medium 7 9) The correct structure for methyl bicyclo[2.2.2]octane-1-carboxylate is: a) I b) II c) III d) IV e) V Answer: D Topic: Nomenclature Section: 17.2 Difficulty Level: Medium 10) A correct name for is: O O a) 2-Methylbutyl 2-methylbutanoate b) 2-Methylbutyl 3-methylbutanoate c) 3-Methylbutyl isovalerate d) Isopentyl isovalerate e) Isopentyl isobutyrate Answer: B Topic: Nomenclature Section: 17.2 Difficulty Level: Medium 8 11) The correct structure for ethyl 3-methylbutanoate is: O O O O O O I II III O O O O IV V a) I b) II c) III d) IV e) V Answer: D Topic: Nomenclature Section: 17.2 Difficulty Level: Medium 12) What is the IUPAC name for Cl Cl O a) -Chlorovaleryl chloride b) 2-Chloropentanoyl chloride c) 1-Chloropentanoyl chloride d) 1,2-Dichloropentanal e) 1-Chloro-1-butanecarbonyl chloride Answer: B Topic: Nomenclature Section: 17.2 Difficulty Level: Medium 9 13) The correct structure for ethyl 2-chloropentanoyl chloride is: O O O OH Cl OH Cl OH Cl I II III O Cl O Cl Cl IV V Cl a) I b) II c) III d) IV e) V Answer: E Topic: Nomenclature Section: 17.2 Difficulty Level: Medium 14) What is the IUPAC name for O O a) -Dimethylbutyl acetate- b) -Dimethyl-4-oxoethanal c) -Dimethylbutyl methanoate d) -Dimethylbutyl methylate e) -Dimethylbutyl formylate Answer: C Topic: Nomenclature Section: 17.2 Difficulty Level: Medium 10 15) The correct structure for -methylbutyl methanoate is: O O O H O H O H O I II III O O O O IV V a) I b) II c) III d) IV e) V Answer: C Topic: Nomenclature Section: 17.2 Difficulty Level: Medium 11 16) Which of the following structures is N-benzyl-N-propyl-2,3-dimethylbutanamide? H N N N O O O I II III N N O O IV V a) I b) II c) III d) IV e) V Answer: E Topic: Nomenclature Section: 17.2 Difficulty Level: Medium 12 17) Which of the following structures is 3,4-dimethylpentyl chloroformate? H O Cl O Cl O O Cl O O I II III O Cl O O Cl O IV V a) I b) II c) III d) IV e) V Answer: E Topic: Nomenclature Section: 17.9 Difficulty Level: Hard 18) Which compound would be the strongest acid? a) CHCl2CH2CH2CO2H b) ClCH2CHClCH2CO2H c) CH3CCl2CH2CO2H d) CH3CHClCHClCO2H e) CH3CH2CCl2CO2H Answer: E Topic: Acidity Section: 17.2 Difficulty Level: Easy 13 19) Which compound would be the weakest acid? a) CHCl2CH2CH2CO2H b) ClCH2CHClCH2CO2H c) CH3CCl2CH2CO2H d) CH3CHClCHClCO2H e) CH3CH2CCl2CO2H Answer: A Topic: Acidity Section: 17.2 Difficulty Level: Easy 20) Which compound would be the strongest acid? a) 4,4-dichlorobutanoic acid b) 3,4-dichlorobutanoic acid c) 3,3-dichlorobutanoic acid d) 2,3-dichlorobutanoic acid e) 2,2-dichlorobutanoic acid Answer: E Topic: Acidity Section: 17.2 Difficulty Level: Medium 21) Which compound would be expected to have the lowest pKa? a) 4,4-dichlorobutanoic acid b) 3,4-dichlorobutanoic acid c) 3,3-dichlorobutanoic acid d) 2,3-dichlorobutanoic acid e) 2,2-dichlorobutanoic acid Answer: E Topic: Acidity Section: 17.2 Difficulty Level: Medium 14 22) Which compound would be expected to have the highest pKa? a) 4,4-dichlorobutanoic acid b) 3,4-dichlorobutanoic acid c) 3,3-dichlorobutanoic acid d) 2,3-dichlorobutanoic acid e) 2,2-dichlorobutanoic acid Answer: A Topic: Acidity Section: 17.2 Difficulty Level: Medium 23) Which compound would be the weakest acid? a) 4,4-dichlorobutanoic acid b) 3,4-dichlorobutanoic acid c) 3,3-dichlorobutanoic acid d) 2,3-dichlorobutanoic acid e) 2,2-dichlorobutanoic acid Answer: A Topic: Acidity Section: 17.2 Difficulty Level: Medium 24) Which compound would be the strongest acid? a) water b) acetic acid c) ethane d) acetylene e) ethanol Answer: B Topic: Acidity Section: 17.2 Difficulty Level: Medium 15 25) Which compound would be the weakest acid? a) water b) acetic acid c) ethane d) acetylene e) ethanol Answer: C Topic: Acidity Section: 17.2 Difficulty Level: Medium 26) Which compound would be expected to have the highest pKa? a) water b) acetic acid c) ethane d) acetylene e) ethanol Answer: C Topic: Acidity Section: 17.2 Difficulty Level: Medium 27) Which compound would be expected to have the lowest pKa? a) water b) acetic acid c) ethane d) acetylene e) ethanol Answer: B Topic: Acidity Section: 17.2 Difficulty Level: Medium 16 28) In which of the following sequences are the compounds listed in order of decreasing acidity? a) CH3COOH > H2O > CH3CH2OH > HC CH > NH3 b) CH3CH2OH > CH3COOH > H2O > HC CH > NH3 c) CH3COOH > CH3CH2OH > H2O > NH3 > HC CH d) H2O > CH3COOH > CH3CH2OH > HC CH > NH3 e) CH3CH2OH > H2O > CH3COOH > HC CH > NH3 Answer: A Topic: Acidity Section: 17.2 Difficulty Level: Medium 29) In which of the following sequences are the compounds listed in order of increasing acidity? a) NH3 < HC CH < CH3CH2OH < H2O < CH3COOH b) CH3CH2OH < NH3 < H2O < HC CH < CH3COOH c) CH3COOH < CH3CH2OH < H2O < NH3 < HC CH d) H2O < CH3COOH < CH3CH2OH < HC CH < NH3 e) NH3 < H2O < CH3COOH < HC CH < CH3CH2OH Answer: A Topic: Acidity Section: 17.2 Difficulty Level: Medium 30) In which of the following sequences are the compounds listed in order of decreasing acidity? a) CH3COOH > H2O > PhOH > HC CH > NH3 b) PhOH > CH3COOH > H2O > HC CH > NH3 c) CH3COOH > PhOH > H2O > HC CH > NH3 d) H2O > CH3COOH > PhOH > HC CH > NH3 e) PhOH > H2O > CH3COOH > HC CH > NH3 Answer: C Topic: Acidity Section: 17.2 17 Difficulty Level: Medium 31) In which of the following sequences are the compounds listed in order of decreasing acidity? a) PhCOOH > H2O > PhOH > PhCH2OH > PhH b) PhCOOH > PhOH > H2O > PhCH2OH > PhH c) PhH > H2O > PhOH > PhCH2OH > PhCOOH d) PhOH > H2O > PhCOOH > PhCH2OH > PhH e) PhCOOH > H2O > PhOH > PhH > PhCH2OH Answer: B Topic: Acidity Section: 17.2 Difficulty Level: Medium 32) Which of the following would be the strongest acid? Cl CO2H CO2H CO2H Cl Cl Cl I II III CO2H CO2H Cl Cl Cl IV V a) I b) II c) III d) IV e) V Answer: C Topic: Acidity Section: 17.2 Difficulty Level: Medium 18 33) Which of the following would be the weakest acid? Cl CO2H CO2H CO2H Cl Cl Cl I II III CO2H CO2H Cl Cl Cl IV V a) I b) II c) III d) IV e) V Answer: A Topic: Acidity Section: 17.2 Difficulty Level: Medium 34) Which of the following would be the strongest acid? F CO2H CO2H CO2H F F F I II III CO2H CO2H F F F IV V a) I b) II c) III d) IV e) V Answer: C 19 Topic: Acidity Section: 17.2 Difficulty Level: Medium 35) Which of the following would be the strongest acid? F CO2H CO2H CO2H Cl F F I II III CO2H CO2H F Cl Cl IV V a) I b) II c) III d) IV e) V Answer: C Topic: Acidity Section: 17.2 Difficulty Level: Medium 36) Which of the following would be the strongest acid? NO2 CO2H CO2H CO2H O2N O2N NO2 I II III CO2H CO2H NO2 O2N NO2 IV V a) I b) II c) III d) IV e) V Answer: C 20 Topic: Acidity Section: 17.2 Difficulty Level: Medium 37) Which of the following would be the strongest acid? CH3 CO2H CO2H CO2H H3C H3C CH3 I II III CO2H CO2H CH3 H3C CH3 IV V a) I b) II c) III d) IV e) V Answer: A Topic: Acidity Section: 17.2 Difficulty Level: Medium 38) Which of the following would be the strongest acid? OCH3 CO2H CO2H CO2H H3CO H3CO OCH3 I II III CO2H CO2H OCH3 H3CO OCH3 IV V a) I b) II c) III d) IV e) V Answer: A a) I b) II c) III d) IV e) V Answer: D Topic: Nomenclature Section: 17.2 Difficulty Level: Medium 2 4) The correct structure for bicyclo[2.2.2]octane-2-carboxylic acid is: a) I b) II c) III d) IV e) V Answer: B Topic: Nomenclature Section: 17.2 Difficulty Level: Medium 3 5) The correct structure for bicyclo[1.1.0]butane-2-carboxylic acid is: a) I b) II c) III d) IV e) V Answer: E Topic: Nomenclature Section: 17.2 Difficulty Level: Medium 4 6) The correct structure for methyl bicyclo[1.1.1]pentane-2-carboxylate is: a) I b) II c) III d) IV e) V Answer: D Topic: Nomenclature Section: 17.2 Difficulty Level: Medium 5 7) The correct structure for methyl bicyclo[2.2.2]octane-2-carboxylate is: a) I b) II c) III d) IV e) V Answer: B Topic: Nomenclature Section: 17.2 Difficulty Level: Medium 6 8) The correct structure for methyl bicyclo[1.1.0]butane-2-carboxylate is: a) I b) II c) III d) IV e) V Answer: E Topic: Nomenclature Section: 17.2 Difficulty Level: Medium 7 9) The correct structure for methyl bicyclo[2.2.2]octane-1-carboxylate is: a) I b) II c) III d) IV e) V Answer: D Topic: Nomenclature Section: 17.2 Difficulty Level: Medium 10) A correct name for is: O O a) 2-Methylbutyl 2-methylbutanoate b) 2-Methylbutyl 3-methylbutanoate c) 3-Methylbutyl isovalerate d) Isopentyl isovalerate e) Isopentyl isobutyrate Answer: B Topic: Nomenclature Section: 17.2 Difficulty Level: Medium 8 11) The correct structure for ethyl 3-methylbutanoate is: O O O O O O I II III O O O O IV V a) I b) II c) III d) IV e) V Answer: D Topic: Nomenclature Section: 17.2 Difficulty Level: Medium 12) What is the IUPAC name for Cl Cl O a) -Chlorovaleryl chloride b) 2-Chloropentanoyl chloride c) 1-Chloropentanoyl chloride d) 1,2-Dichloropentanal e) 1-Chloro-1-butanecarbonyl chloride Answer: B Topic: Nomenclature Section: 17.2 Difficulty Level: Medium 9 13) The correct structure for ethyl 2-chloropentanoyl chloride is: O O O OH Cl OH Cl OH Cl I II III O Cl O Cl Cl IV V Cl a) I b) II c) III d) IV e) V Answer: E Topic: Nomenclature Section: 17.2 Difficulty Level: Medium 14) What is the IUPAC name for O O a) -Dimethylbutyl acetate- b) -Dimethyl-4-oxoethanal c) -Dimethylbutyl methanoate d) -Dimethylbutyl methylate e) -Dimethylbutyl formylate Answer: C Topic: Nomenclature Section: 17.2 Difficulty Level: Medium 10 15) The correct structure for -methylbutyl methanoate is: O O O H O H O H O I II III O O O O IV V a) I b) II c) III d) IV e) V Answer: C Topic: Nomenclature Section: 17.2 Difficulty Level: Medium 11 16) Which of the following structures is N-benzyl-N-propyl-2,3-dimethylbutanamide? H N N N O O O I II III N N O O IV V a) I b) II c) III d) IV e) V Answer: E Topic: Nomenclature Section: 17.2 Difficulty Level: Medium 12 17) Which of the following structures is 3,4-dimethylpentyl chloroformate? H O Cl O Cl O O Cl O O I II III O Cl O O Cl O IV V a) I b) II c) III d) IV e) V Answer: E Topic: Nomenclature Section: 17.9 Difficulty Level: Hard 18) Which compound would be the strongest acid? a) CHCl2CH2CH2CO2H b) ClCH2CHClCH2CO2H c) CH3CCl2CH2CO2H d) CH3CHClCHClCO2H e) CH3CH2CCl2CO2H Answer: E Topic: Acidity Section: 17.2 Difficulty Level: Easy 13 19) Which compound would be the weakest acid? a) CHCl2CH2CH2CO2H b) ClCH2CHClCH2CO2H c) CH3CCl2CH2CO2H d) CH3CHClCHClCO2H e) CH3CH2CCl2CO2H Answer: A Topic: Acidity Section: 17.2 Difficulty Level: Easy 20) Which compound would be the strongest acid? a) 4,4-dichlorobutanoic acid b) 3,4-dichlorobutanoic acid c) 3,3-dichlorobutanoic acid d) 2,3-dichlorobutanoic acid e) 2,2-dichlorobutanoic acid Answer: E Topic: Acidity Section: 17.2 Difficulty Level: Medium 21) Which compound would be expected to have the lowest pKa? a) 4,4-dichlorobutanoic acid b) 3,4-dichlorobutanoic acid c) 3,3-dichlorobutanoic acid d) 2,3-dichlorobutanoic acid e) 2,2-dichlorobutanoic acid Answer: E Topic: Acidity Section: 17.2 Difficulty Level: Medium 14 22) Which compound would be expected to have the highest pKa? a) 4,4-dichlorobutanoic acid b) 3,4-dichlorobutanoic acid c) 3,3-dichlorobutanoic acid d) 2,3-dichlorobutanoic acid e) 2,2-dichlorobutanoic acid Answer: A Topic: Acidity Section: 17.2 Difficulty Level: Medium 23) Which compound would be the weakest acid? a) 4,4-dichlorobutanoic acid b) 3,4-dichlorobutanoic acid c) 3,3-dichlorobutanoic acid d) 2,3-dichlorobutanoic acid e) 2,2-dichlorobutanoic acid Answer: A Topic: Acidity Section: 17.2 Difficulty Level: Medium 24) Which compound would be the strongest acid? a) water b) acetic acid c) ethane d) acetylene e) ethanol Answer: B Topic: Acidity Section: 17.2 Difficulty Level: Medium 15 25) Which compound would be the weakest acid? a) water b) acetic acid c) ethane d) acetylene e) ethanol Answer: C Topic: Acidity Section: 17.2 Difficulty Level: Medium 26) Which compound would be expected to have the highest pKa? a) water b) acetic acid c) ethane d) acetylene e) ethanol Answer: C Topic: Acidity Section: 17.2 Difficulty Level: Medium 27) Which compound would be expected to have the lowest pKa? a) water b) acetic acid c) ethane d) acetylene e) ethanol Answer: B Topic: Acidity Section: 17.2 Difficulty Level: Medium 16 28) In which of the following sequences are the compounds listed in order of decreasing acidity? a) CH3COOH > H2O > CH3CH2OH > HC CH > NH3 b) CH3CH2OH > CH3COOH > H2O > HC CH > NH3 c) CH3COOH > CH3CH2OH > H2O > NH3 > HC CH d) H2O > CH3COOH > CH3CH2OH > HC CH > NH3 e) CH3CH2OH > H2O > CH3COOH > HC CH > NH3 Answer: A Topic: Acidity Section: 17.2 Difficulty Level: Medium 29) In which of the following sequences are the compounds listed in order of increasing acidity? a) NH3 < HC CH < CH3CH2OH < H2O < CH3COOH b) CH3CH2OH < NH3 < H2O < HC CH < CH3COOH c) CH3COOH < CH3CH2OH < H2O < NH3 < HC CH d) H2O < CH3COOH < CH3CH2OH < HC CH < NH3 e) NH3 < H2O < CH3COOH < HC CH < CH3CH2OH Answer: A Topic: Acidity Section: 17.2 Difficulty Level: Medium 30) In which of the following sequences are the compounds listed in order of decreasing acidity? a) CH3COOH > H2O > PhOH > HC CH > NH3 b) PhOH > CH3COOH > H2O > HC CH > NH3 c) CH3COOH > PhOH > H2O > HC CH > NH3 d) H2O > CH3COOH > PhOH > HC CH > NH3 e) PhOH > H2O > CH3COOH > HC CH > NH3 Answer: C Topic: Acidity Section: 17.2 17 Difficulty Level: Medium 31) In which of the following sequences are the compounds listed in order of decreasing acidity? a) PhCOOH > H2O > PhOH > PhCH2OH > PhH b) PhCOOH > PhOH > H2O > PhCH2OH > PhH c) PhH > H2O > PhOH > PhCH2OH > PhCOOH d) PhOH > H2O > PhCOOH > PhCH2OH > PhH e) PhCOOH > H2O > PhOH > PhH > PhCH2OH Answer: B Topic: Acidity Section: 17.2 Difficulty Level: Medium 32) Which of the following would be the strongest acid? Cl CO2H CO2H CO2H Cl Cl Cl I II III CO2H CO2H Cl Cl Cl IV V a) I b) II c) III d) IV e) V Answer: C Topic: Acidity Section: 17.2 Difficulty Level: Medium 18 33) Which of the following would be the weakest acid? Cl CO2H CO2H CO2H Cl Cl Cl I II III CO2H CO2H Cl Cl Cl IV V a) I b) II c) III d) IV e) V Answer: A Topic: Acidity Section: 17.2 Difficulty Level: Medium 34) Which of the following would be the strongest acid? F CO2H CO2H CO2H F F F I II III CO2H CO2H F F F IV V a) I b) II c) III d) IV e) V Answer: C 19 Topic: Acidity Section: 17.2 Difficulty Level: Medium 35) Which of the following would be the strongest acid? F CO2H CO2H CO2H Cl F F I II III CO2H CO2H F Cl Cl IV V a) I b) II c) III d) IV e) V Answer: C Topic: Acidity Section: 17.2 Difficulty Level: Medium 36) Which of the following would be the strongest acid? NO2 CO2H CO2H CO2H O2N O2N NO2 I II III CO2H CO2H NO2 O2N NO2 IV V a) I b) II c) III d) IV e) V Answer: C 20 Topic: Acidity Section: 17.2 Difficulty Level: Medium 37) Which of the following would be the strongest acid? CH3 CO2H CO2H CO2H H3C H3C CH3 I II III CO2H CO2H CH3 H3C CH3 IV V a) I b) II c) III d) IV e) V Answer: A Topic: Acidity Section: 17.2 Difficulty Level: Medium 38) Which of the following would be the strongest acid? OCH3 CO2H CO2H CO2H H3CO H3CO OCH3 I II III CO2H CO2H OCH3 H3CO OCH3 IV V a) I b) II c) III d) IV e) V Answer: A [Show More]
Last updated: 3 years ago
Preview 1 out of 118 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$5.50
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Apr 13, 2021
Number of pages
118
Written in
All
Additional information
This document has been written for:
Uploaded
Apr 13, 2021
Downloads
0
Views
81