Physics > QUESTIONS & ANSWERS > PHYSICS 30>Assignment 16( With 2 parts:Part One: Cathode Rays and Thomson’s Experiment and Part Tw (All)
PHYSICS 30>Assignment 16( With 2 parts:Part One: Cathode Rays and Thomson’s Experiment and Part Two: The Millikan Experiment) < VERIFIED SCREENSHOTED ANSWERS >
Document Content and Description Below
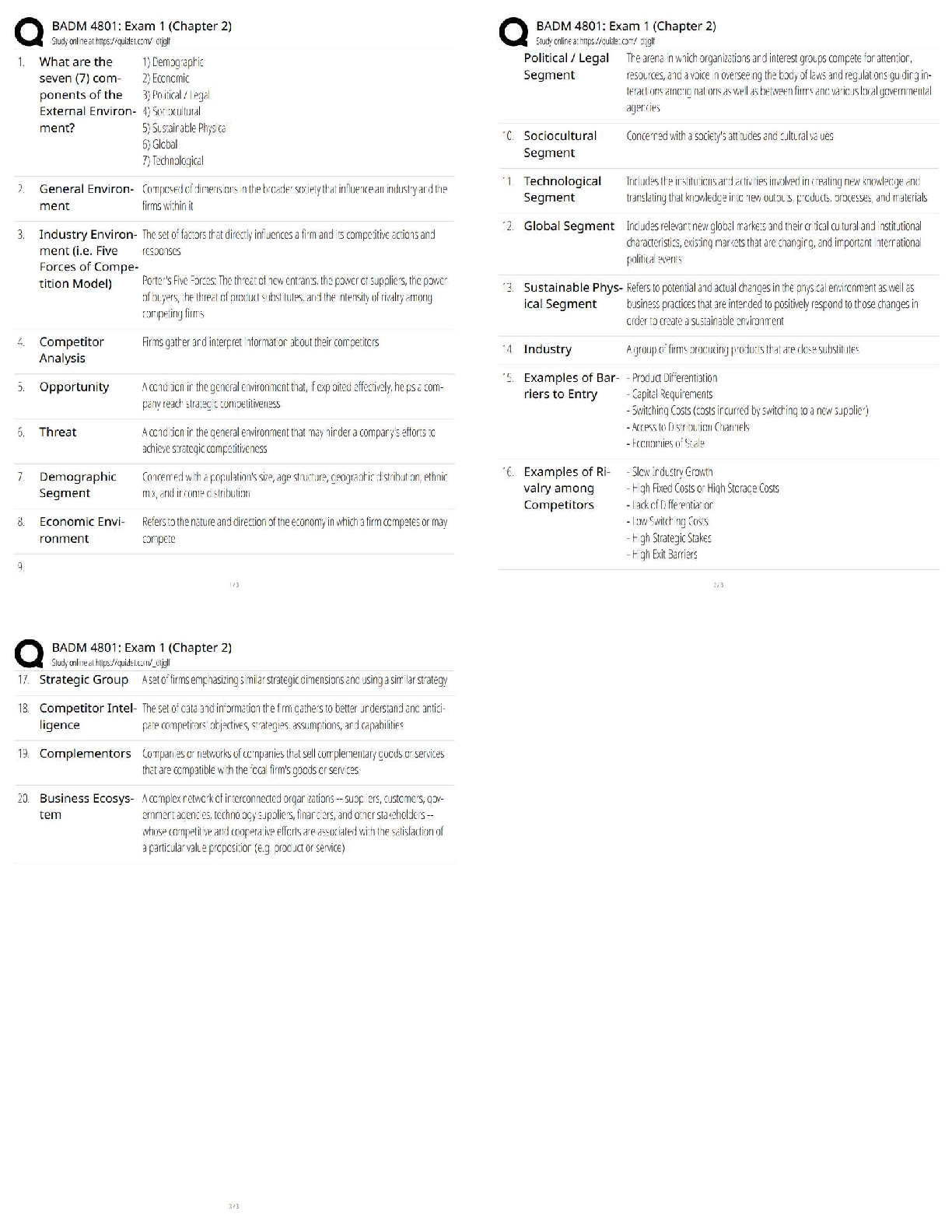
Physics 30: Assignment 16 1 52 Marks Total ASSIGNMENT 16 Part One: Cathode Rays and Thomson’s Experiment Part One of this assignment is worth 25 marks. The value of each question is noted in ... the left margin in parenthesis. Note: The answer areas will expand to fit the length of your response. (2) 1. What was the evidence that the cathode rays were particles with charge and mass? Answer: Cathode rays would cause the glass to glow when they stuck the side of a tube and they could be deflected by a magnetic field. Therefore, it was suggested that the rays must be streams of negatively charged particles. (3) 2. What is the charge to mass ratio of a particle travelling 3.60 x 106 m/s that is deflected in an arc or radius 7.40 cm as it travels through a magnetic field of 0.610 T? Answer: (3) 3. Some positively charged particles passed un-deflected through perpendicular magneticPhysics 30: Assignment 16 2 and electric fields. If the magnetic field strength was 0.650 T and the electric field strength was 2.10 x 105 N/C, what was the speed of the particles? Answer: (3) 4. Alpha particles travel through a magnetic field of 0.360 T and are deflected in an arc of 0.0820 m. Assuming the alpha particles are travelling perpendicular to the field, what is the kinetic energy of an individual alpha particle? Answer:Physics 30: Assignment 16 3 (3) 5. Using the charge of an electron (determined by Millikan in another experiment to be 1.60 x 10-19 C) and the charge to mass ratio of the electron (determined by Thomson to be 1.76 x 1011 C/kg), calculate the mass of an electron. Answer: 6. A particle accelerated by a potential difference enters a velocity selector which consists of perpendicular magnetic and electric fields. The particle travels straight when the magnetic field is 0.400 T and the electric field is 6.30 × 105 V/m. Once the electric field is turned off, a sensor determines that the radius or the particle’s path is 4.11 cm. (3) a. What is the charge-to-mass ratio of this particle? Answer: (4) b. Calculate the charge-to-mass ratio of an alpha particle, electron and proton. Identify the particles used in the experiment from the calculations. Hint: check your data booklet for the charges and masses. Answer: [Show More]
Last updated: 3 years ago
Preview 1 out of 11 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Also available in bundle (1)
Click Below to Access Bundle(s)

Sir Winston Churchill High School ,Physics 30: Assignments<FROM ASSIGMENT 10, 11, , 14, 16, 17>ALL ANSWERS VERIFIED, 100% CORRECT
Physics 30: Assignment 10 Part One: Electromagnetic Radiation Part One of this assignment is worth 11 marks. The value of each question is noted in parentheses in the left margin. Note: The answer...
By Expert Tutor 4 years ago
$35
6
Reviews( 0 )
$7.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Apr 14, 2021
Number of pages
11
Written in
All
Additional information
This document has been written for:
Uploaded
Apr 14, 2021
Downloads
0
Views
112