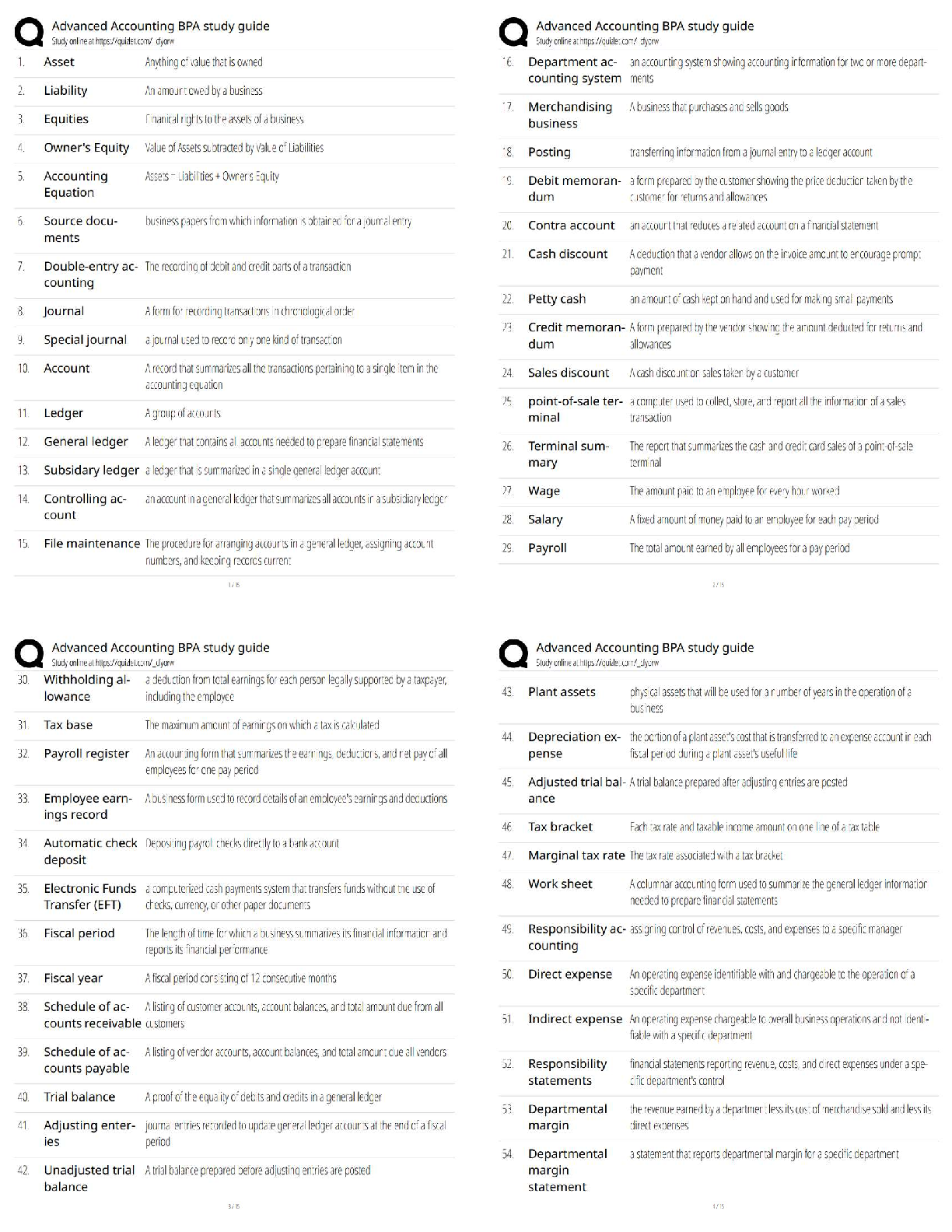
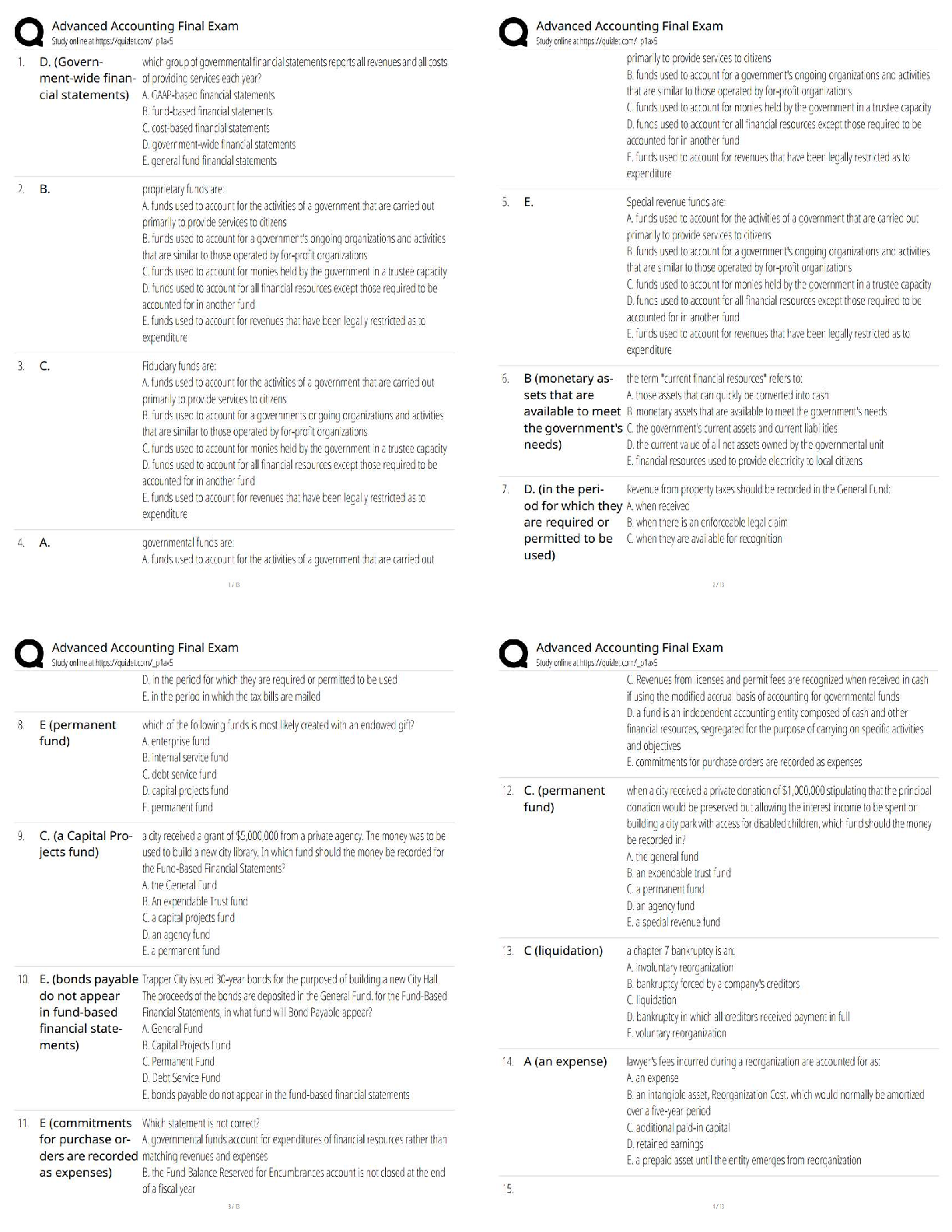
Unit Activity: Chemical Quantities
Task 1: How Airbags Protect Drivers
Part A
Conduct Internet research to learn how airbags work in automobiles. Take notes on your
research. Then write one to two paragraphs explaini
...
Unit Activity: Chemical Quantities
Task 1: How Airbags Protect Drivers
Part A
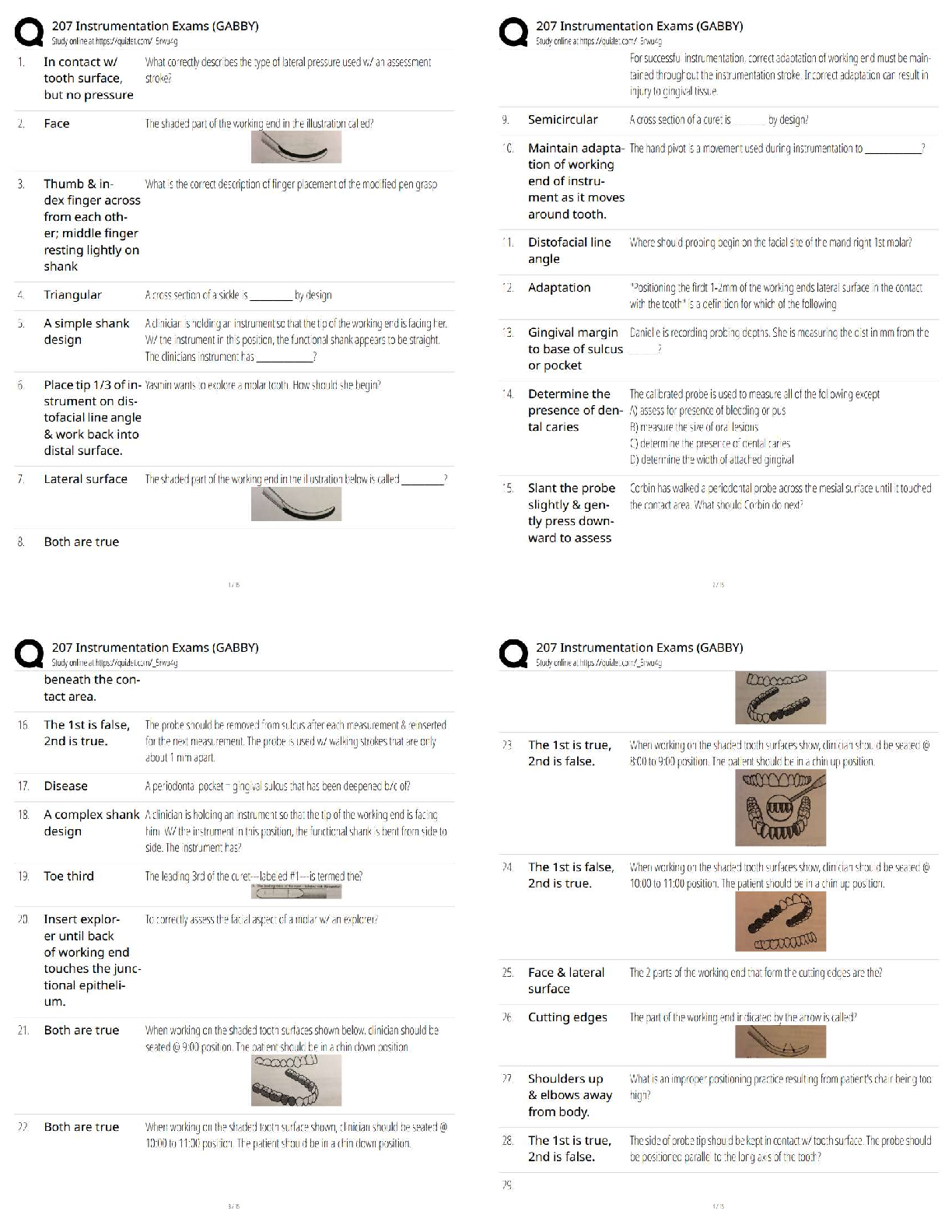
Conduct Internet research to learn how airbags work in automobiles. Take notes on your
research. Then write one to two paragraphs explaining what causes airbags to inflate when a
car is involved in an accident.
Through the processes of working in a car, there is a lot of chemistry involved. Chemistry
involvement examples in a car are the burning of gas for the engine, and the chemical reactions
in the car battery for generating electricity, and the inflation of airbags. Airbags are inflated by
products of chemical reactions. The main chemical source of the airbag inflation is known as
NaN, sodium azide.
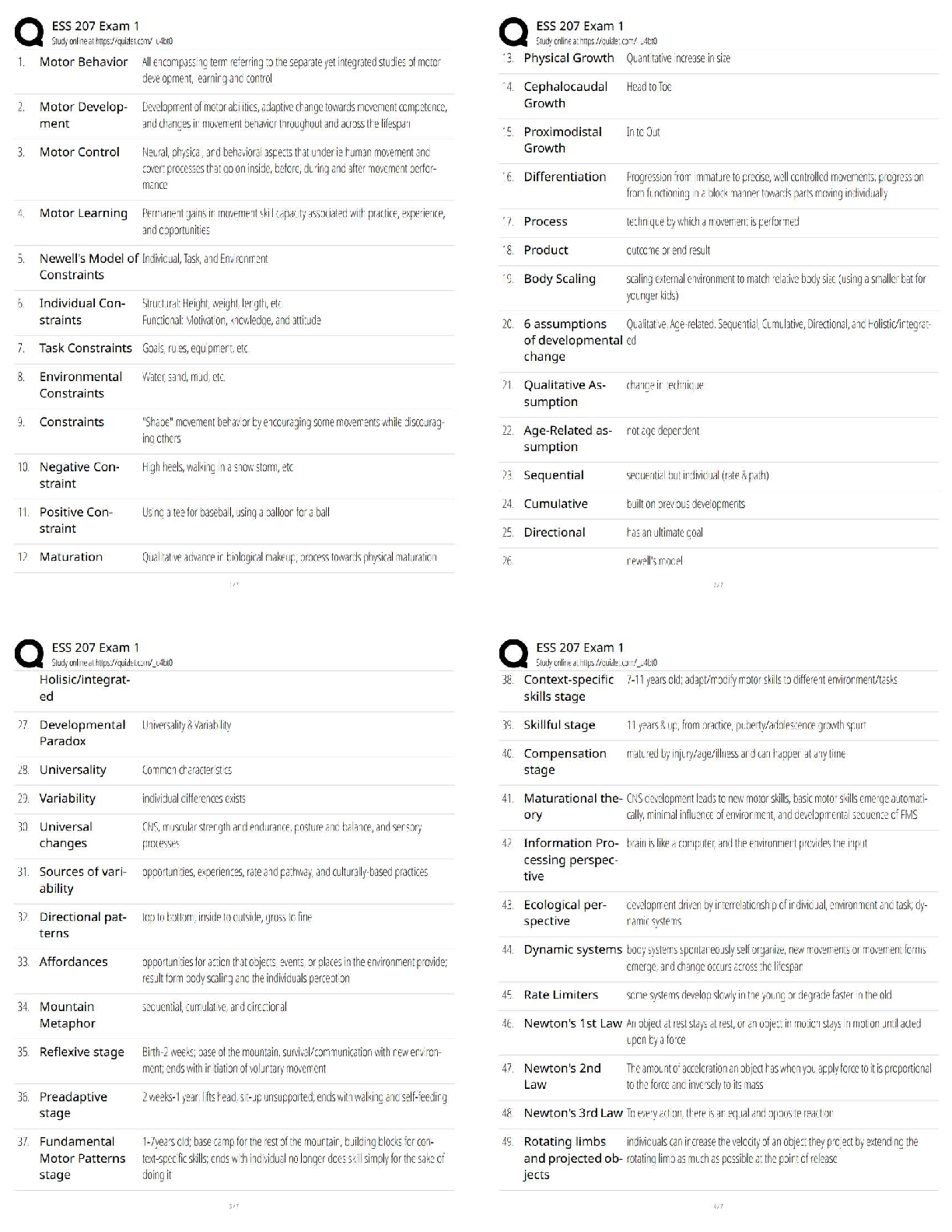
Part B
Sodium azide (NaN
3
) is a substance that can be used to inflate airbags. An electrical impulse
causes the sodium azide to decompose, producing elemental sodium and nitrogen gas. Write
the balanced chemical equation for this reaction.
2NaN
3 → 2Na +3N2
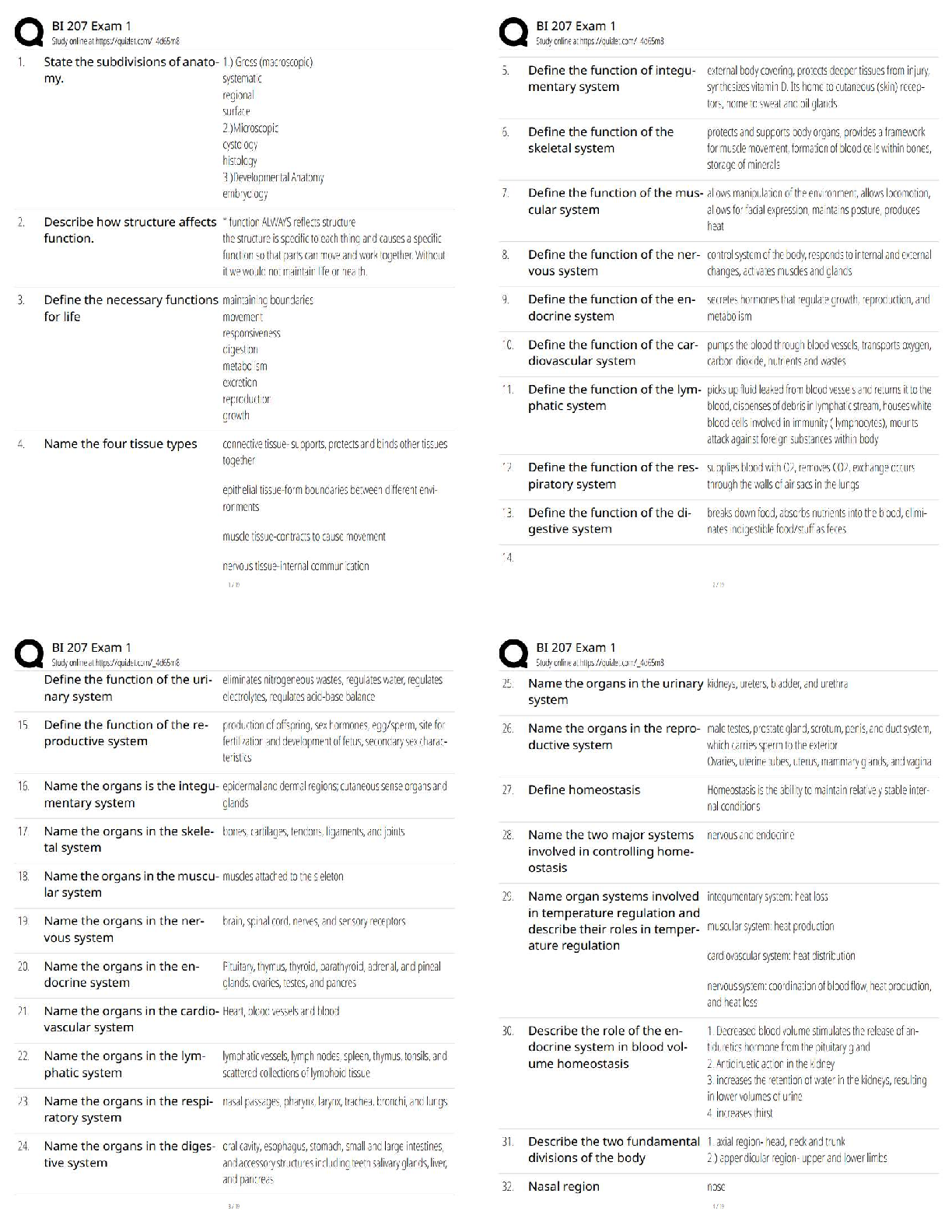
Part C
Now run this simulation that demonstrates the properties of gases. The simulation shows a
container with a lid on top. The lid can be opened and closed by dragging it left and right. On
the left side of the container is a yellow handle. Drag this handle right or left to decrease or
increase the volume of the container. On the top right of the container are a thermometer and a
pressure gauge. Follow these steps to use the simulation, and then answer the question that
follows.
1. Reduce the container to its least volume by dragging the yellow handle as far right as
you can.
2. In the Gas in Chamber menu, add 80 molecules of heavy species to the container.
3. In the Constant Parameter menu, select Pressure.
4. Increase the number of heavy species molecules to 200.
What do you observe when the molecules are increased from 80 to 200 at constant pressure?
When the molecules are increased from 80 to 200 at constant pressure, the temperature
decreases from 299 K to 120 K.Part D
Go back to the simulation and increase the number of molecules of heavy species to 700. What
change do you observe?
When the molecules are increased from 200 to 700 at constant pressure, the temperature
decreases from 120 K to 34 K.
Part E
Based on your observations, relate the container from the simulation to a car airbag. What
would happen to an inflatable airbag if air molecules were to be continuously added to it, similar
to the container in the simulation.
If air molecules were to be continuously added to an inflatable airbag, it would react at the same
constant pressure, just like the container in the simulation, with different temperatures. The
increase of the molecules added means the decrease of the temperature.
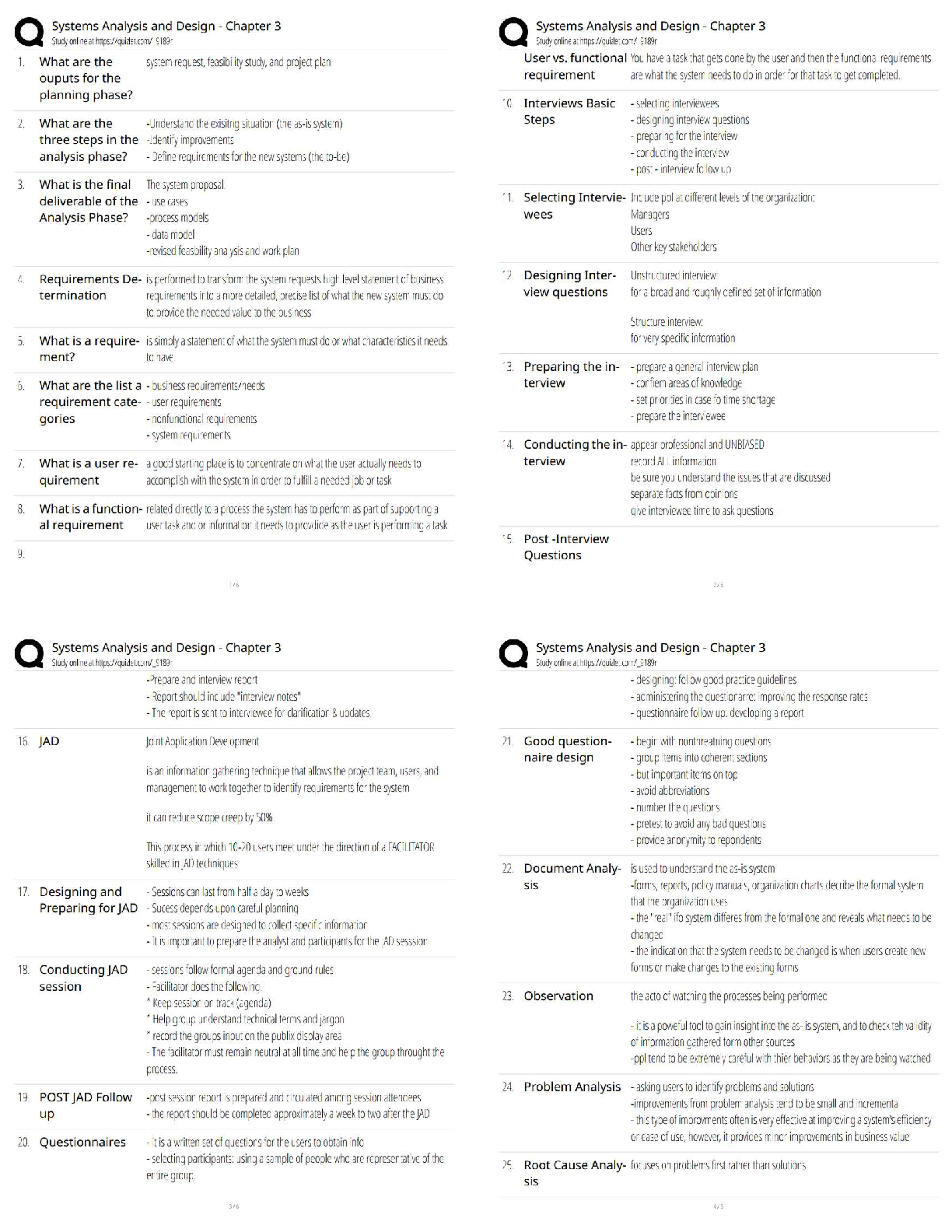
Task 2: Reactants & Products in an Airbag Inflator
Part A:
Deploying an airbag in an automobile is a complex process. Identify at least three steps in the
process. Refer to your research in part A of task 1 if you need help.
1. An impact from a collision will trigger the airbag system. The position of the sensors will
depend on the number of airbags within a vehicle.
2. No matter what type of sensor is used, when the sensor is triggered within the vehicle,
the airbag system will detonate a chemical canister to help in the process of deploying
the airbag.
3. Once the sensors detonate, the nitrogen gas is released in the system. This is what
inflates the airbag.
Part B:
[Show More]