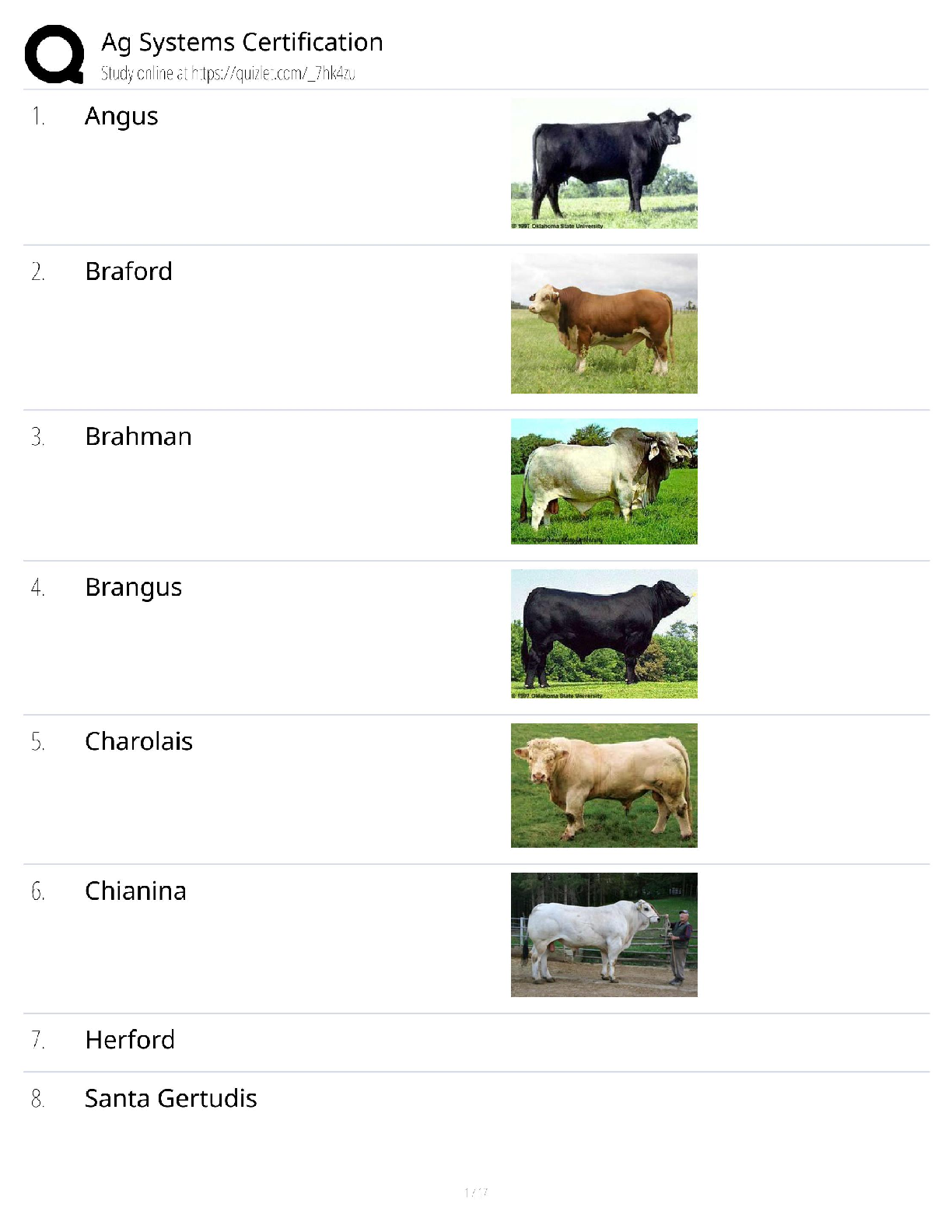
SPED 5311 Final Exam Study Guide | Special Education Foundations | 100% Correct Answers (2025/2026 Updated)
$ 11

LPEC Certification HQP #1 / Lab Pack & Hazardous Waste / 2025 Exam Prep / Score 100% / Study Guide