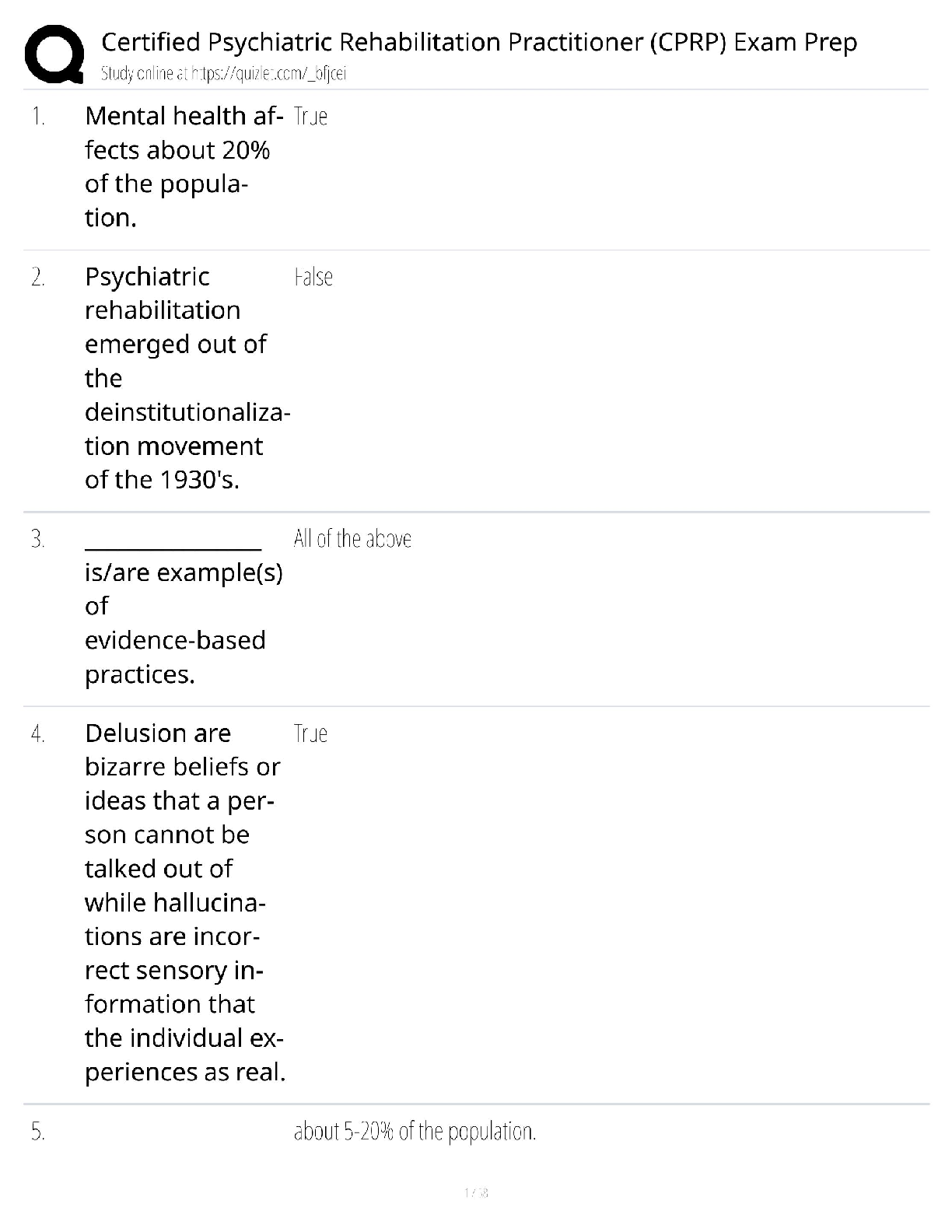
1 The displayed formula of an organic compound is shown below.
What is the systematic name of this organic compound?
A Propyl propanoate
B Propyl butanoate
C Butyl propanoate
D Butyl butanoate
Your answer
[1] Spec
...
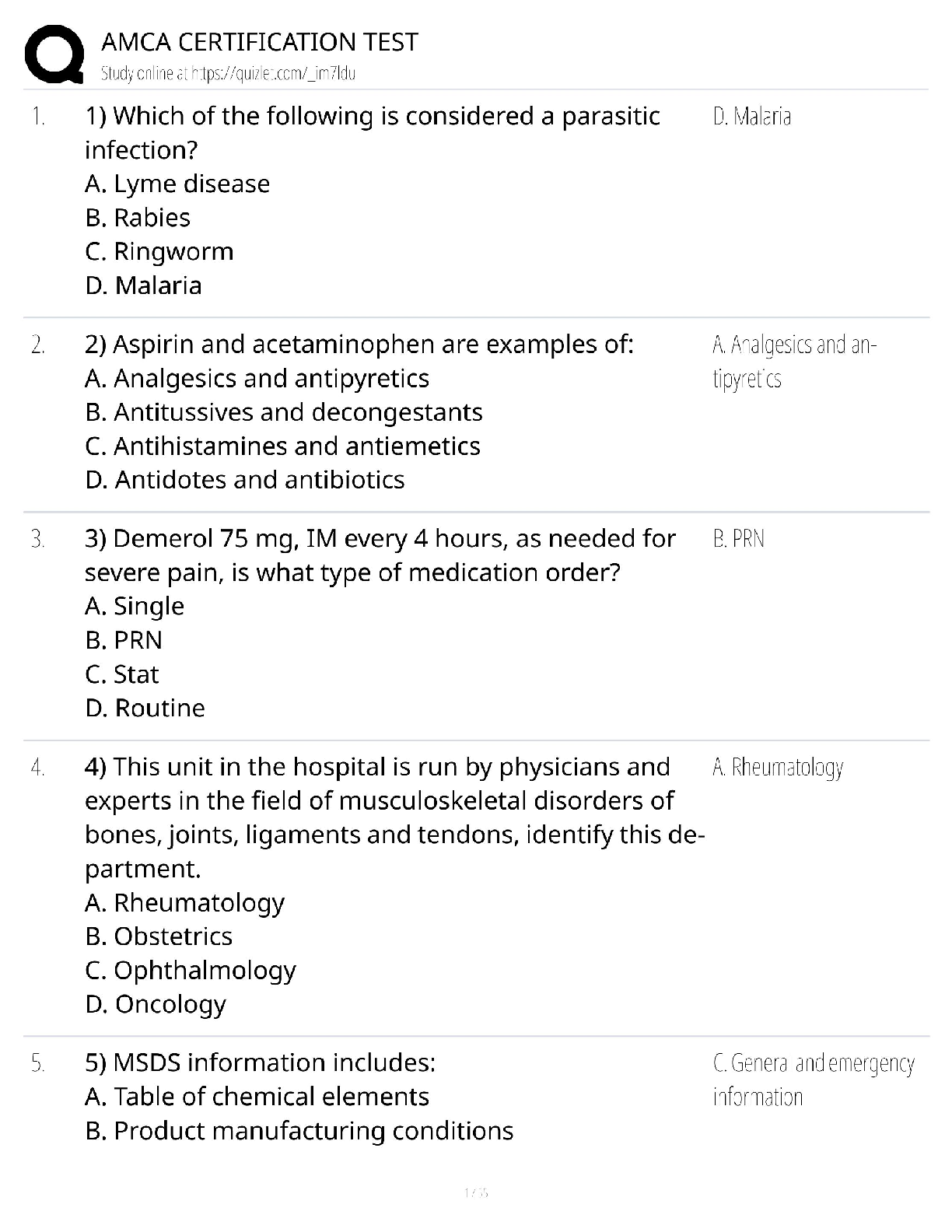
1 The displayed formula of an organic compound is shown below.
What is the systematic name of this organic compound?
A Propyl propanoate
B Propyl butanoate
C Butyl propanoate
D Butyl butanoate
Your answer
[1] Specimen
PMT
3
© OCR 2014 H432/02 Turn over
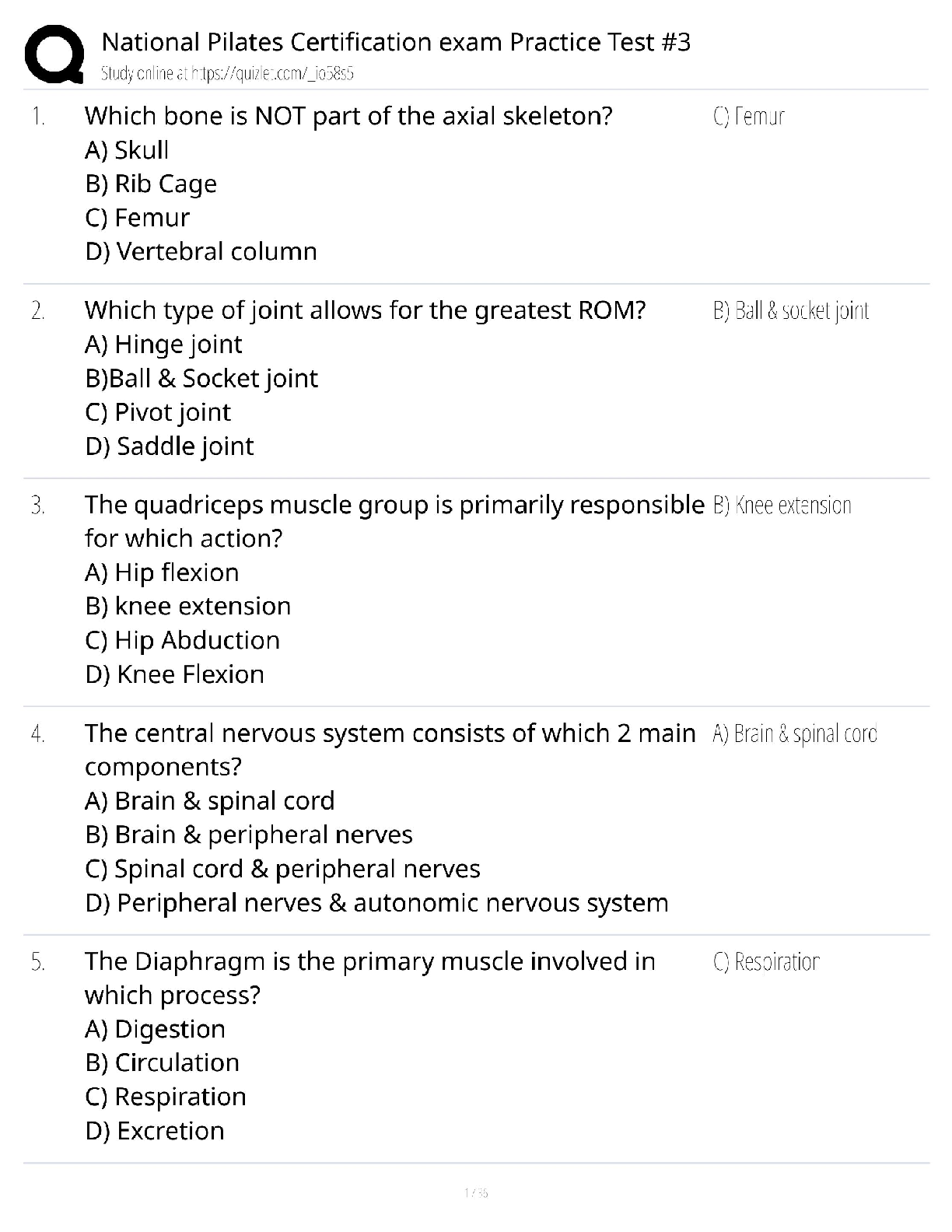
2 Ethanol is oxidised to ethanoic acid using acidified potassium dichromate(IV) solution. The reaction is
heated under reflux using the equipment shown in the diagram below.
What is the reason for heating under reflux?
A to ensure even heating
B to prevent any substances escaping
C to boil the mixture at a higher temperature
D to allow efficient mixing
Your answer
[1]
3 How many stereoisomers are there of CH3CH=CHCH(OH)CH2CH=CH2?
A 2
B 4
C 6
D 8
Your answer
[1]
Specimen
PMT
4
© OCR 2014 H432/02
4 The functional group in an organic compound, W, was identified by carrying out two chemical tests.
The results of the tests are shown below.
Heating with acidified sodium
dichromate(VI)(aq)
Addition of
2,4-dinitrophenylhydrazine(aq)
orange solution turns green yellow/orange precipitate formed
Which compound could be W?
A CH3CH2CH2OH
B CH3COCH3
C CH3CH(OH)CH3
D CH3CH2CHO
Your answer
[1]
5 Complete combustion of 40 cm3 of a gaseous hydrocarbon X requires 240 cm3 of oxygen. 160 cm3 of
carbon dioxide forms. All gas volumes are at room temperature and pressure.
What is the formula of X?
A C4H8
B C4H10
C C6H12
D C6H14
Your answer
[1]
Specimen
PMT
5
© OCR 2014 H432/02 Turn over
6 The boiling point of butan-1-ol is 118 °C. The boiling point of 2-methylpropan-2-ol is 82 °C.
Why is the boiling point of butan-1-ol higher than that of 2-methylpropan-2-ol?
A butan-1-ol has stronger induced dipole–dipole interactions because it has more electrons
B butan-1-ol has stronger induced dipole–dipole interactions because it has a straight-chain
structure
C butan-1-ol can form hydrogen bonds while 2-methylpropan-2-ol cannot
D butan-1-ol is more stable because it is a primary alcohol
Your answer
[1]
7 Hydrogen bromide reacts with 3-methylbut-1-ene.
What is the structure of the major intermediate formed in the mechanism?
A
B
C
D
Your answe
[Show More]