Chemistry > QUESTIONS & ANSWERS > Texas State UniversityCHEMISTRY 1310ChemTaylor2 (All)
Texas State UniversityCHEMISTRY 1310ChemTaylor2
Document Content and Description Below
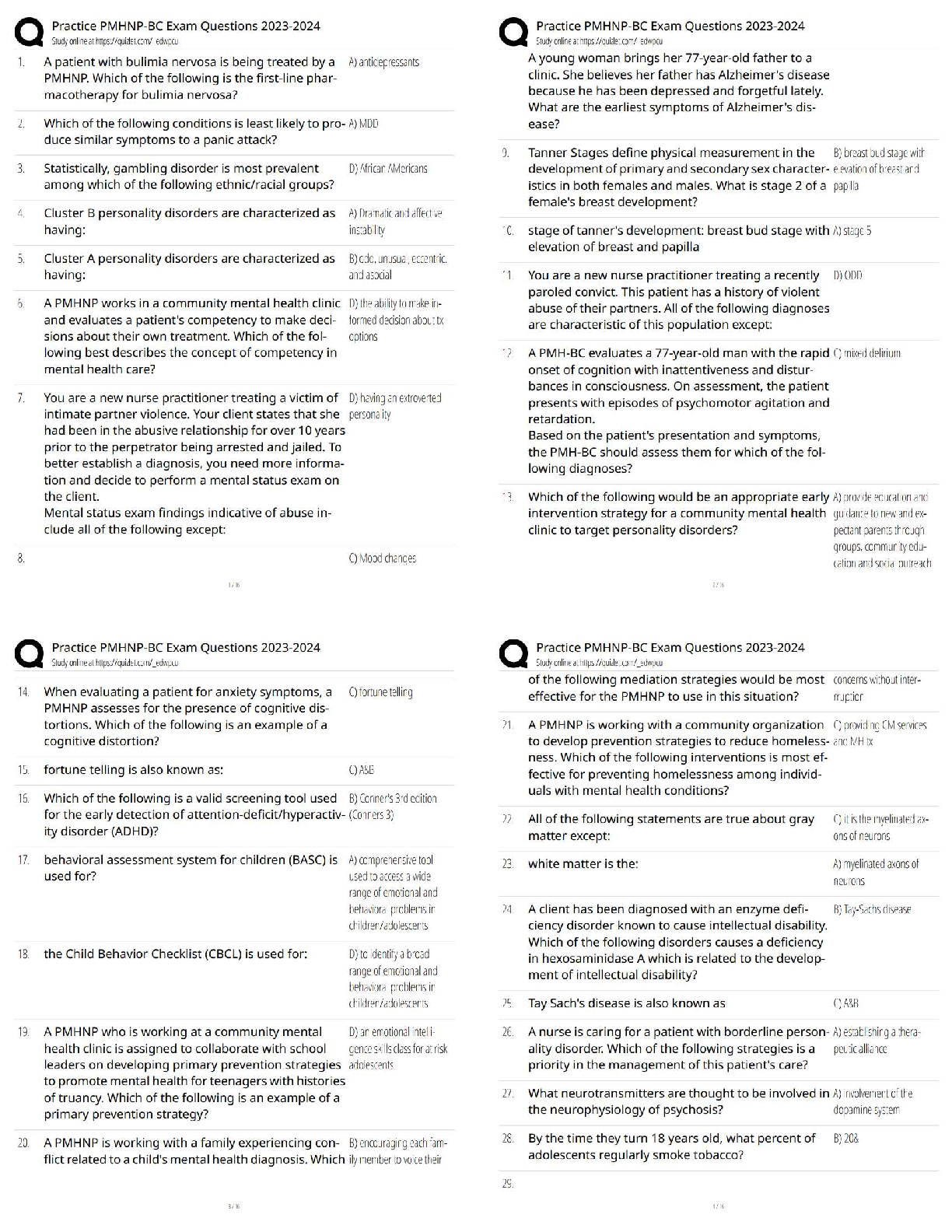
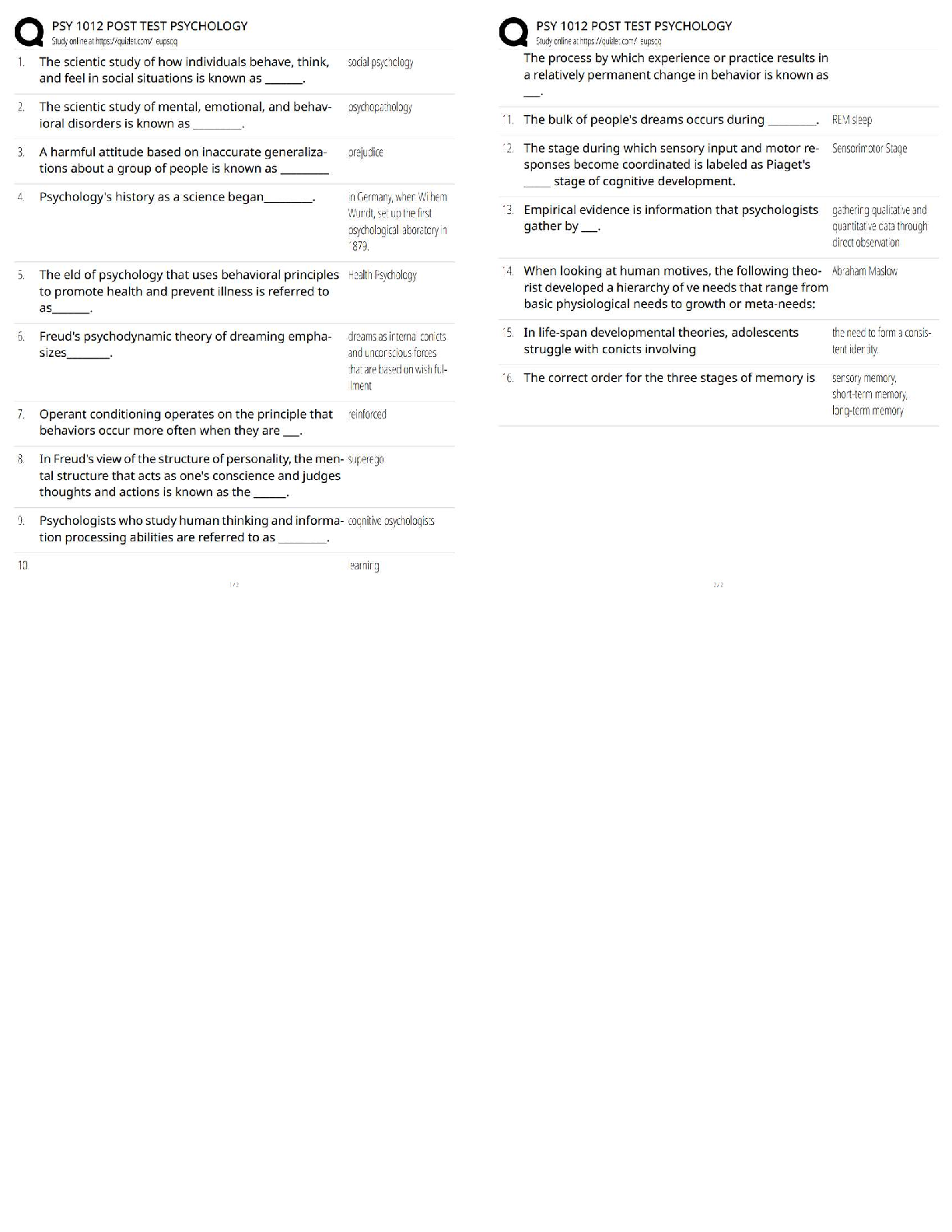
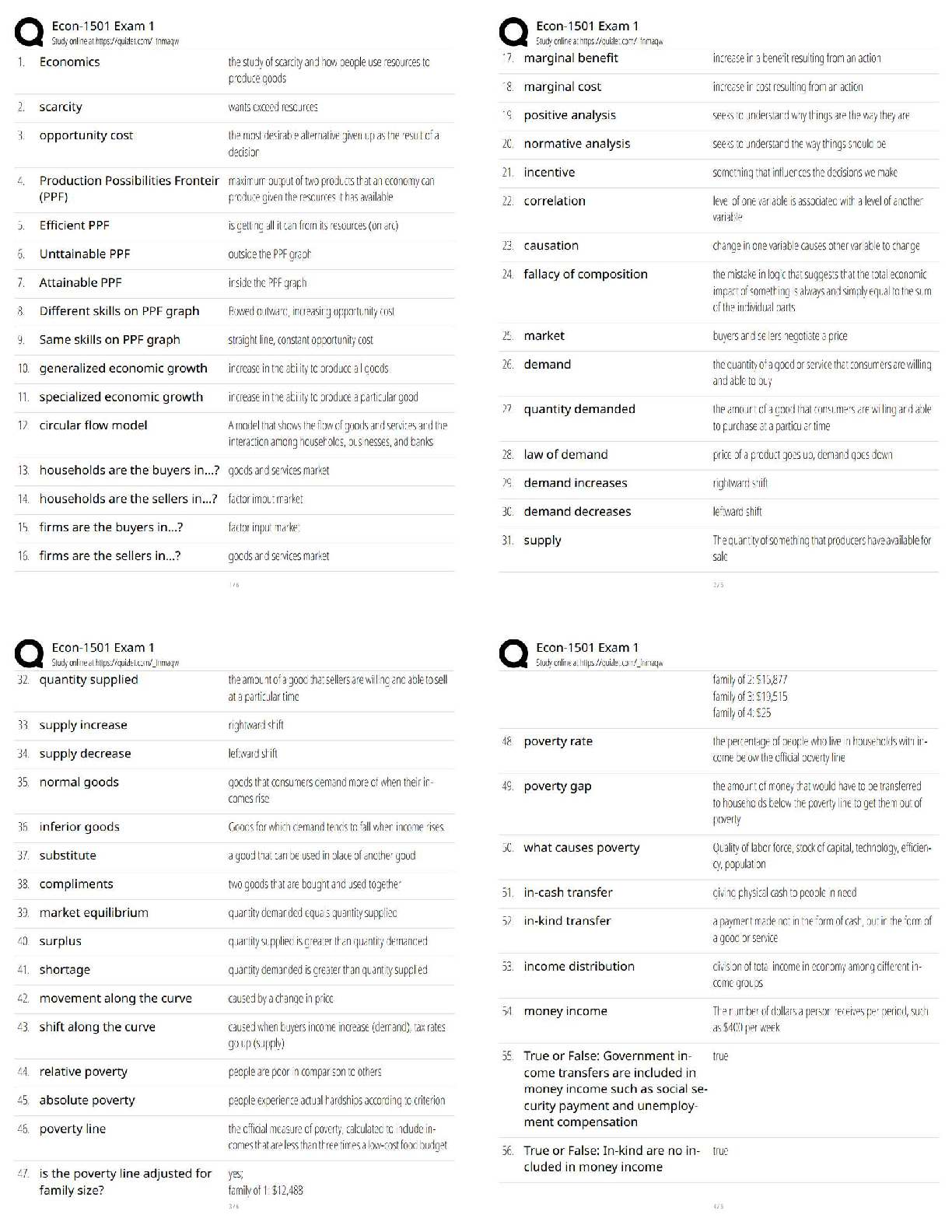
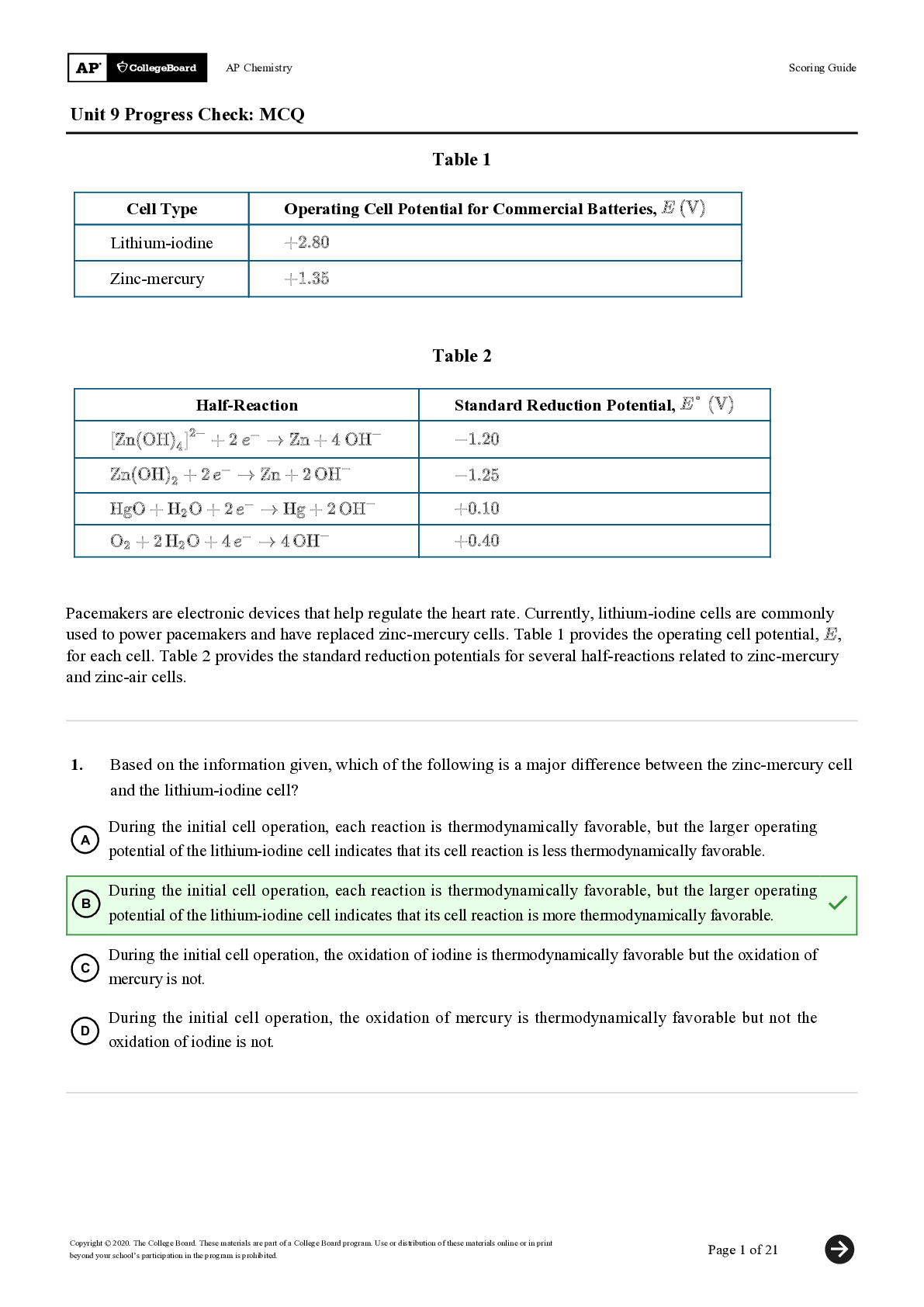
Unit 9 Progress Check: MCQ Copyright © 2020. The College Board. These materials are part of a College Board program. Use or distribution of these materials online or in print beyond your school’s ... participation in the program is prohibited. Page 1 of 21 Table 1 Cell Type Operating Cell Potential for Commercial Batteries, Lithium-iodine Zinc-mercury Table 2 Half-Reaction Standard Reduction Potential, Pacemakers are electronic devices that help regulate the heart rate. Currently, lithium-iodine cells are commonly used to power pacemakers and have replaced zinc-mercury cells. Table 1 provides the operating cell potential, , for each cell. Table 2 provides the standard reduction potentials for several half-reactions related to zinc-mercury and zinc-air cells. 1. Based on the information given, which of the following is a major difference between the zinc-mercury cell and the lithium-iodine cell? A During the initial cell operation, each reaction is thermodynamically favorable, but the larger operating potential of the lithium-iodine cell indicates that its cell reaction is less thermodynamically favorable. B During the initial cell operation, each reaction is thermodynamically favorable, but the larger operating potential of the lithium-iodine cell indicates that its cell reaction is more thermodynamically favorable. C During the initial cell operation, the oxidation of iodine is thermodynamically favorable but the oxidation of mercury is not. D During the initial cell operation, the oxidation of mercury is thermodynamically favorable but not the oxidation of iodine is not.AP Chemistry Scoring Guide Unit 9 Progress Check: MCQ Copyright © 2020. The College Board. These materials are part of a College Board program. Use or distribution of these materials online or in print beyond your school’s participation in the program is prohibited. Page 2 of 21 2. On average, after one year of operation, the potential of a lithium-iodine cell decreases by 1%-2%. Which of the following best helps to explain the cause for the decrease in cell potential? A , and as the cell operates, decreases. B , and as the cell operates, increases. C , and as the cell operates, decreases. D , and as the cell operates, increases. [Show More]
Last updated: 3 years ago
Preview 1 out of 21 pages
Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$9.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
May 15, 2021
Number of pages
21
Written in
All
Additional information
This document has been written for:
Uploaded
May 15, 2021
Downloads
0
Views
76