Reaction Energy GIZMO ( ALL ANSWERS ARE 100% CORRECT )
Document Content and Description Below
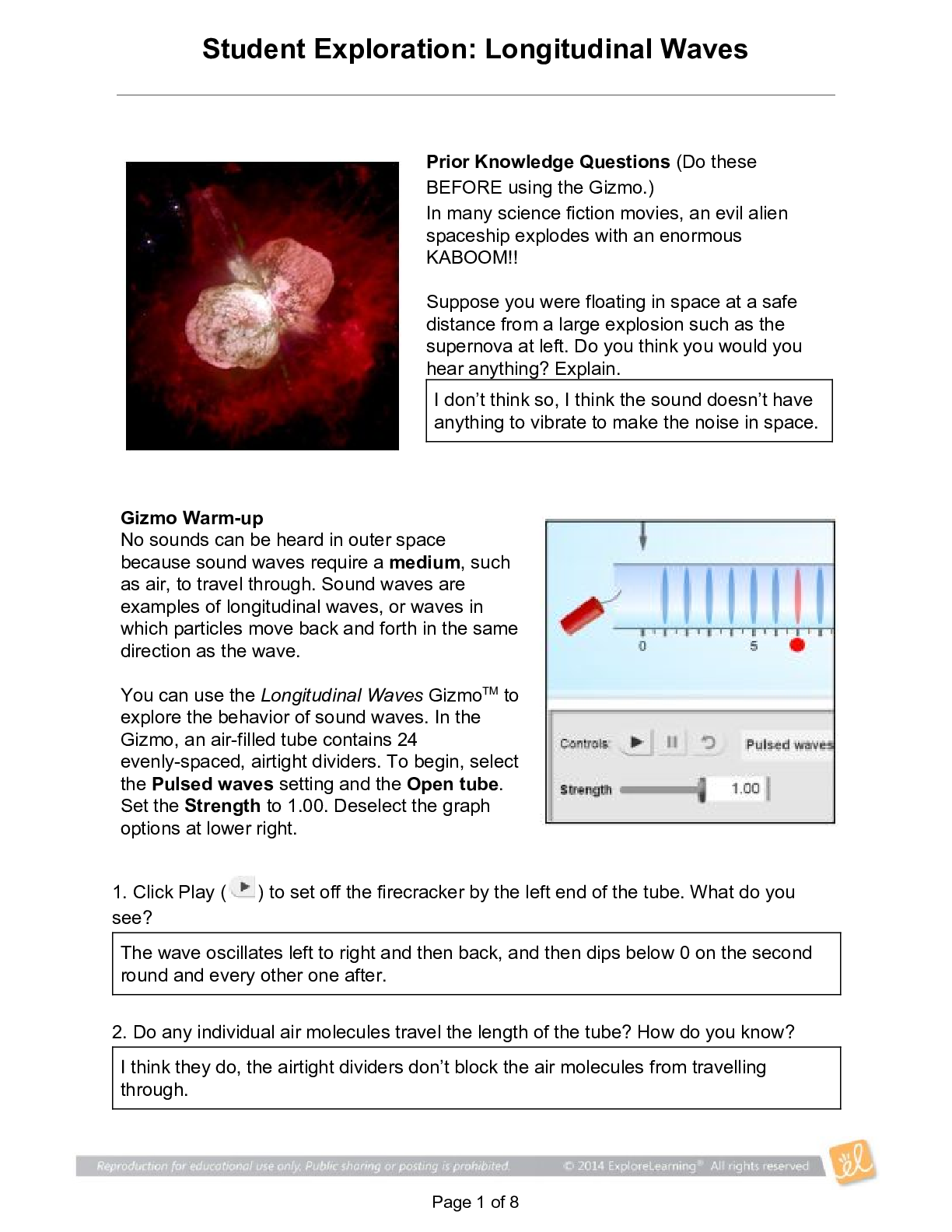
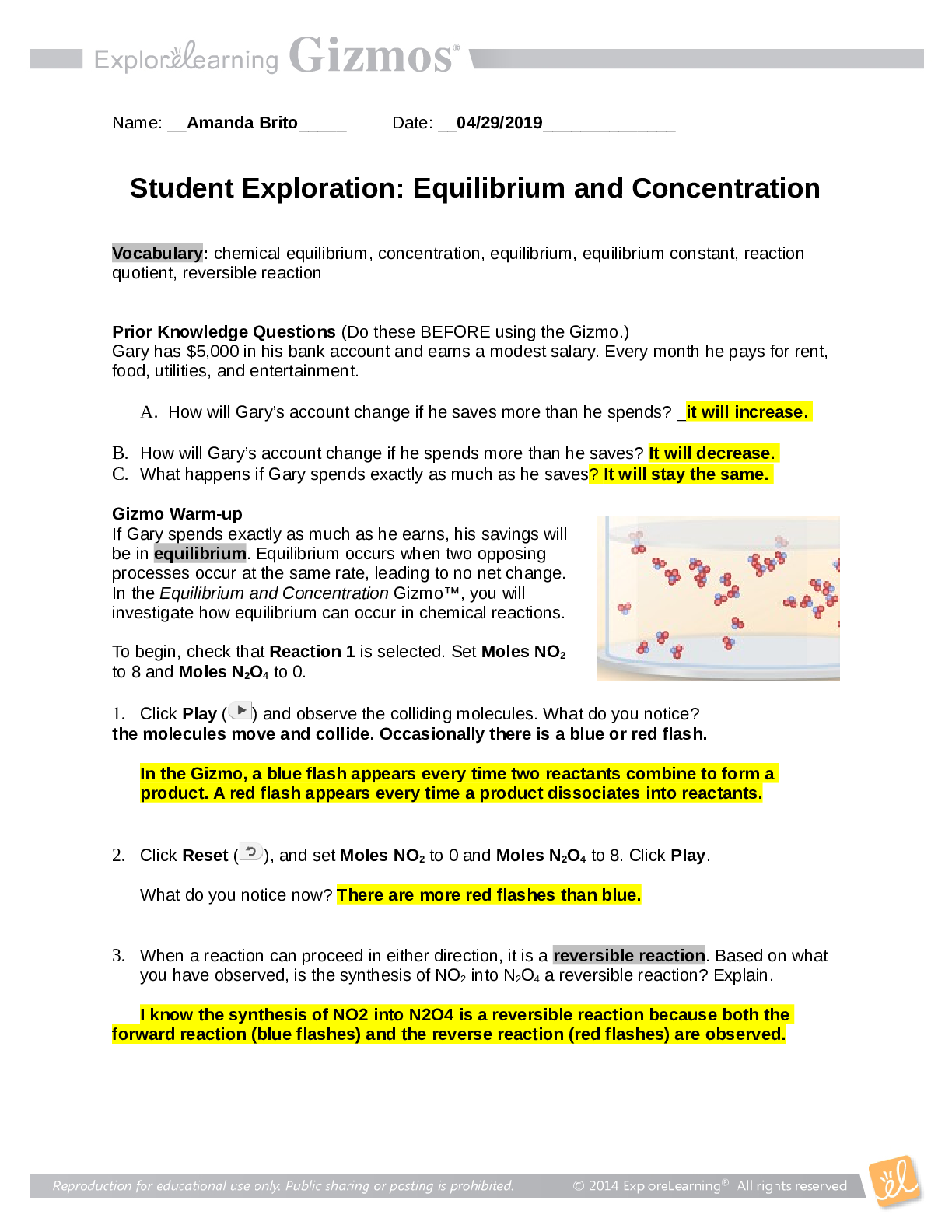
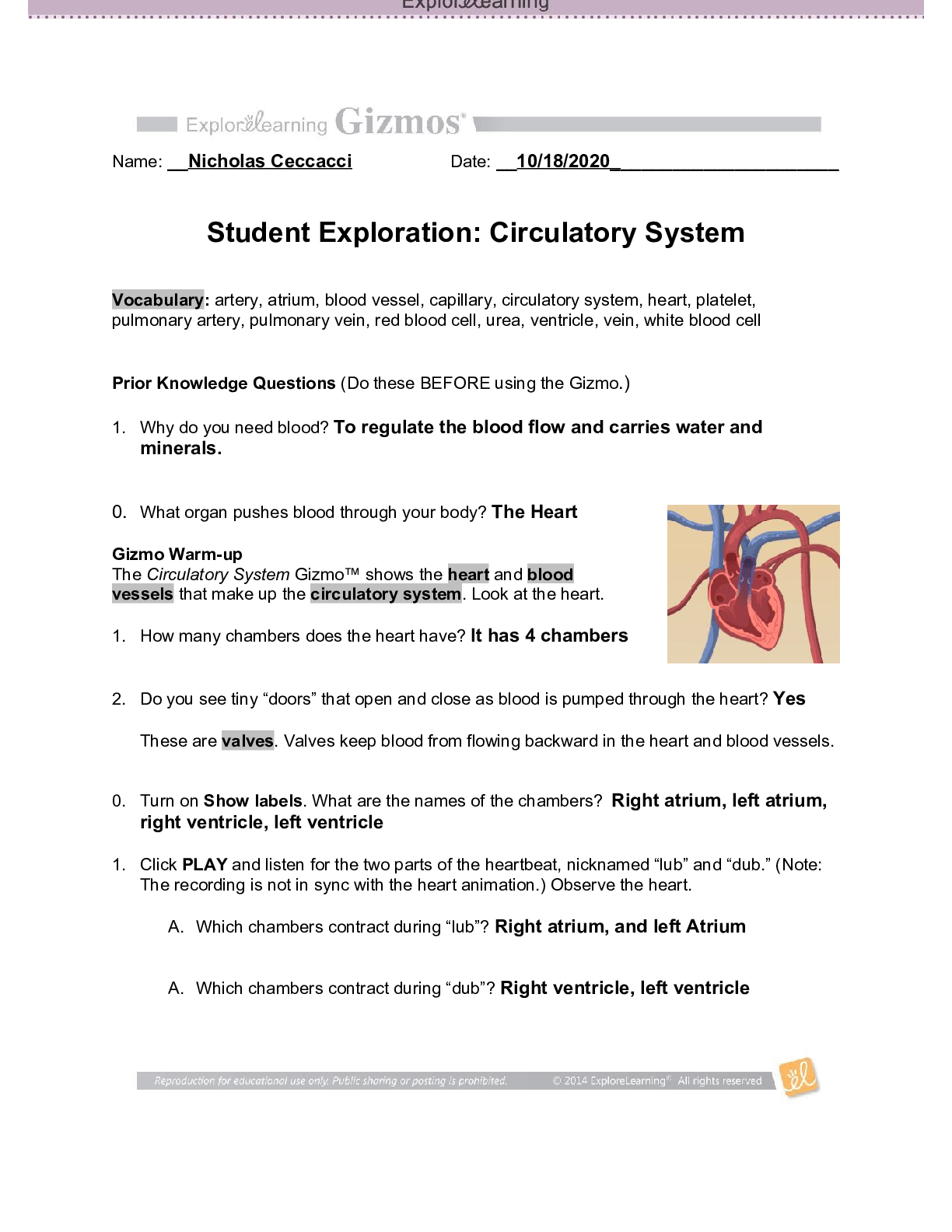
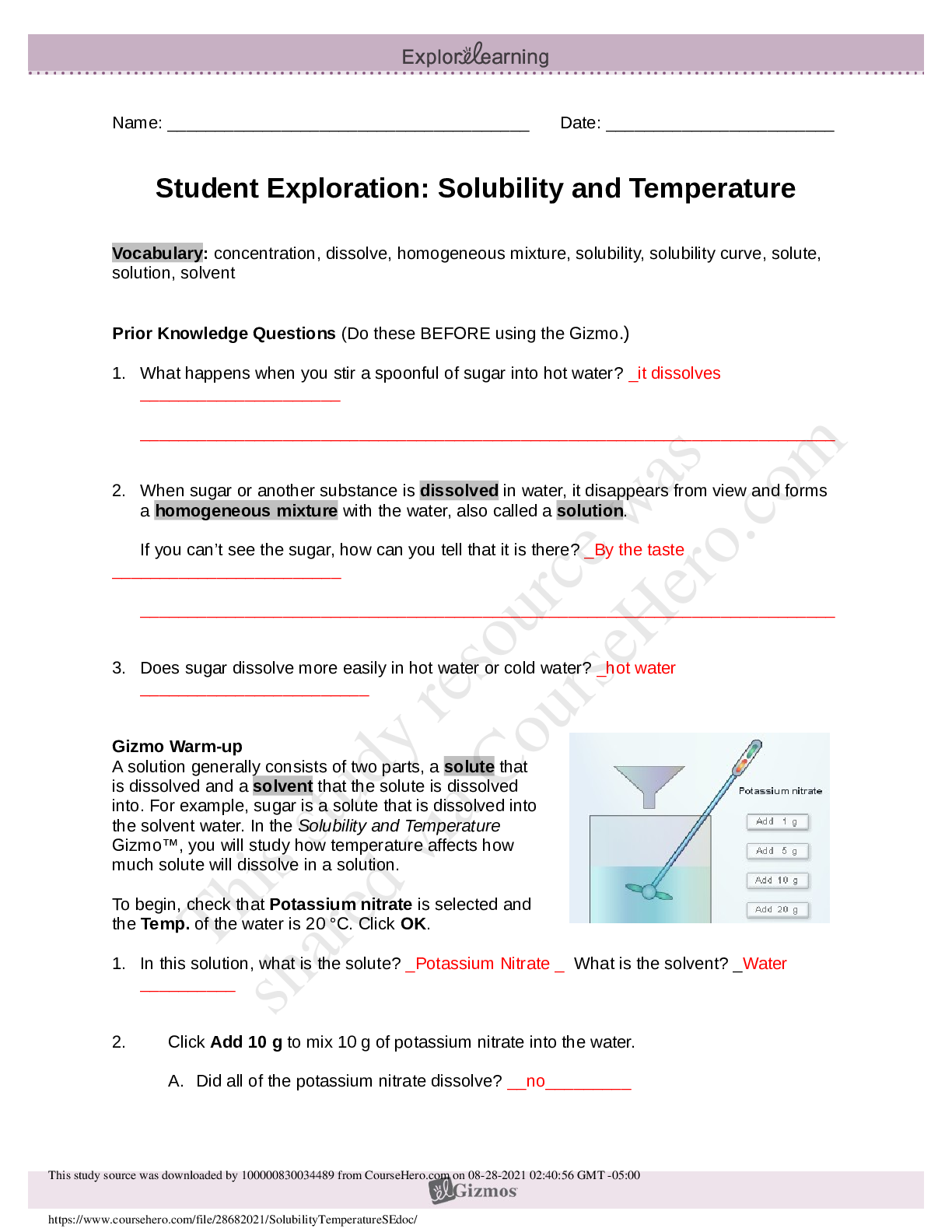
Student Exploration: Reaction Energy Vocabulary: calorimeter, chemical bond, endothermic, enthalpy, exothermic, Hess’s law Prior Knowledge Questions (Do these BEFORE using the Gizmo.) 1. Two magn... ets are stuck together. What might you have to do to get them to separate? Pull them apart 2. Suppose you held two magnets a short distance apart, then let go. What would happen? They would connect 3. Think about the magnets in terms of energy. In which case do you increase the potential energy of the magnets? In which case do you increase the kinetic energy of the magnets? Gizmo Warm-up Just like magnets, atoms of different elements are attracted together to form chemical bonds. Breaking these bonds requires energy. When a new bond forms, energy is released and temperatures rise. In the Reaction Energy Gizmo™, you will explore how the energy of chemical bonding relates to temperature changes that occur during chemical reactions. To begin, check that Reaction 1 and Forward are selected. In this reaction, hydrogen (H2) and oxygen (O2) react to form water (H2O). The reaction takes place inside a device called a calorimeter. Inside the calorimeter, a small chamber holds the reactants. The rest of the calorimeter is filled with water. 1. Click Play ( ). What happens? When I clicked play, all of the separate H2 and O2 molecules formed to create H2O. 2. How does the temperature change? The temperature is increased while the molecules are reacting to form water, and the increase in temperature slows once the molecules are all bonded to create the H2O molecules. The temperature raises from 21.0 Degrees Celsius to 26.0 Degrees Celsius while the molecules were bonding.Activity A: Energy of chemical bonds Get the Gizmo ready: Check that Reaction 1 and Forward are selected. Select the INVESTIGATION tab. Introduction: The heat energy stored in a chemical system is called the enthalpy (H) of the system. When atoms are joined by a chemical bond, energy must be added to pull them apart. This increases the enthalpy of the system. When a chemical bond forms, energy is released as shared electrons move into lower-energy orbitals. This causes the enthalpy to decrease. Question: How can you predict how much energy is released in a chemical reaction? [Show More]
Last updated: 2 years ago
Preview 1 out of 13 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$11.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Jun 11, 2021
Number of pages
13
Written in
Additional information
This document has been written for:
Uploaded
Jun 11, 2021
Downloads
0
Views
200










.png)



.png)