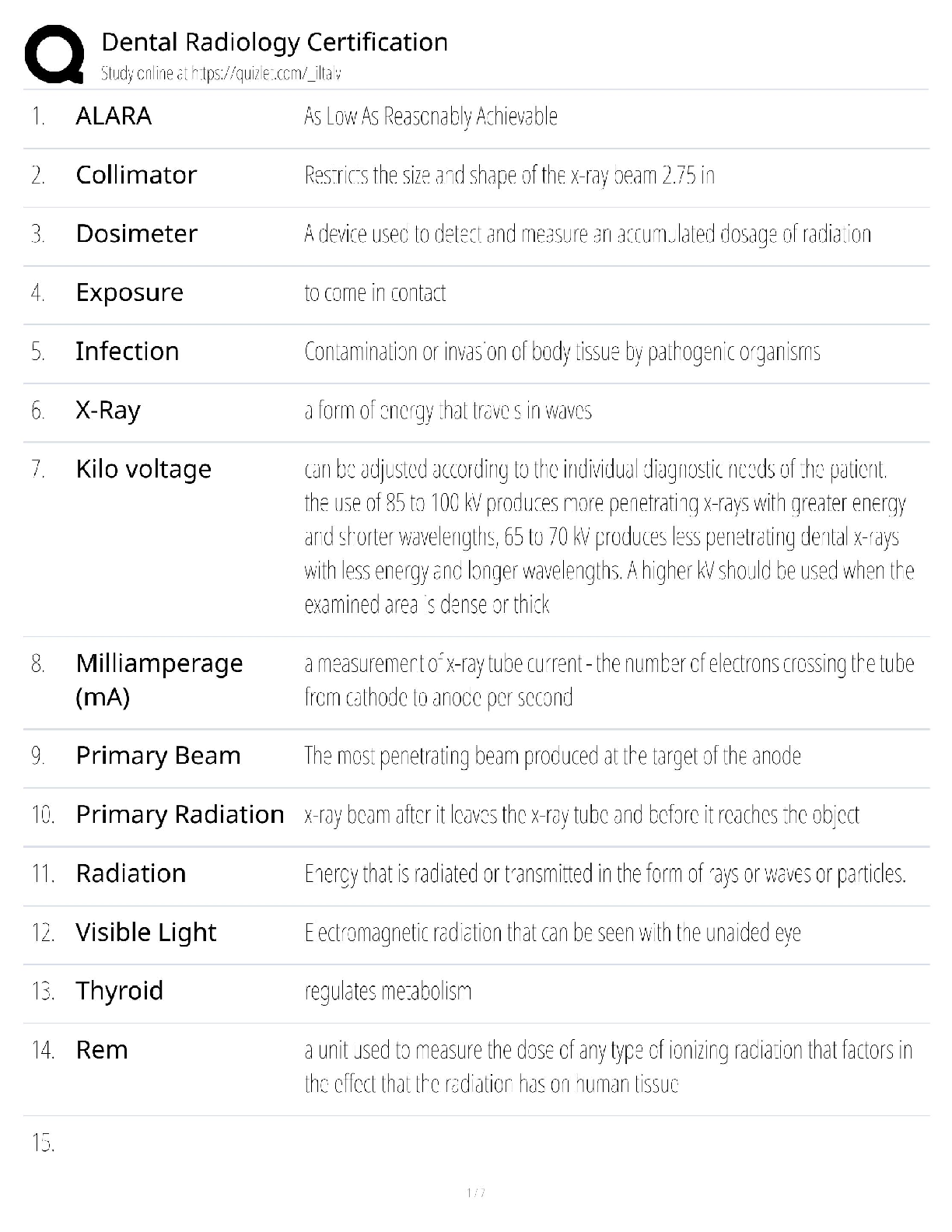
C How to Program, 9e Paul Deitel, Harvey Deitel (Solution Manual)
$ 25
.png)
AQA 2022 PHYSICS AS LEVEL 7407 PAPER 1 QP
$ 8

NATE Exam: Core Essentials Exams Latest Update
$ 12.5
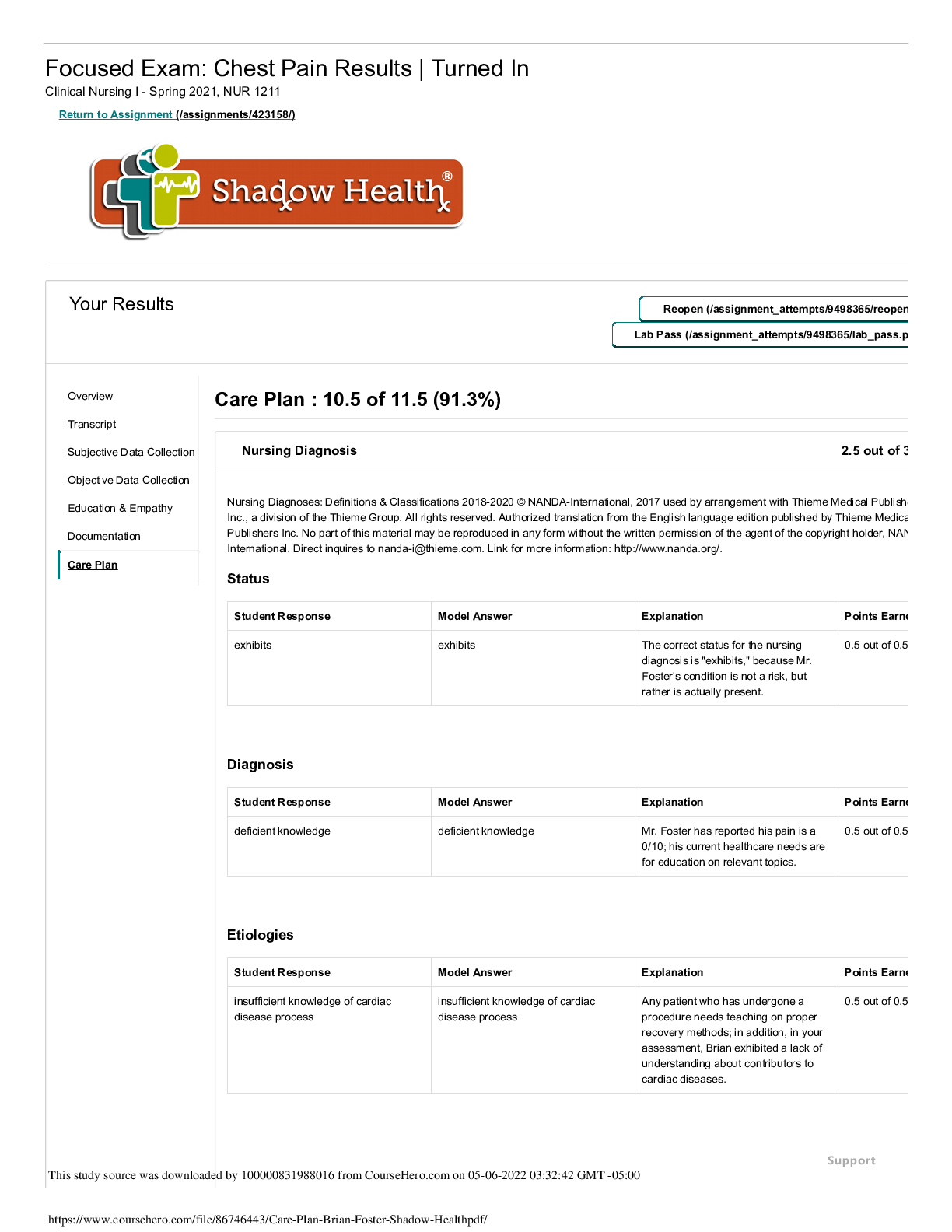
Brian Foster Chest Pain Results: Care Plan Documentation Focused Exam
$ 11

Engineering Economy 7th edition Solutions Manual



.png)





.png)