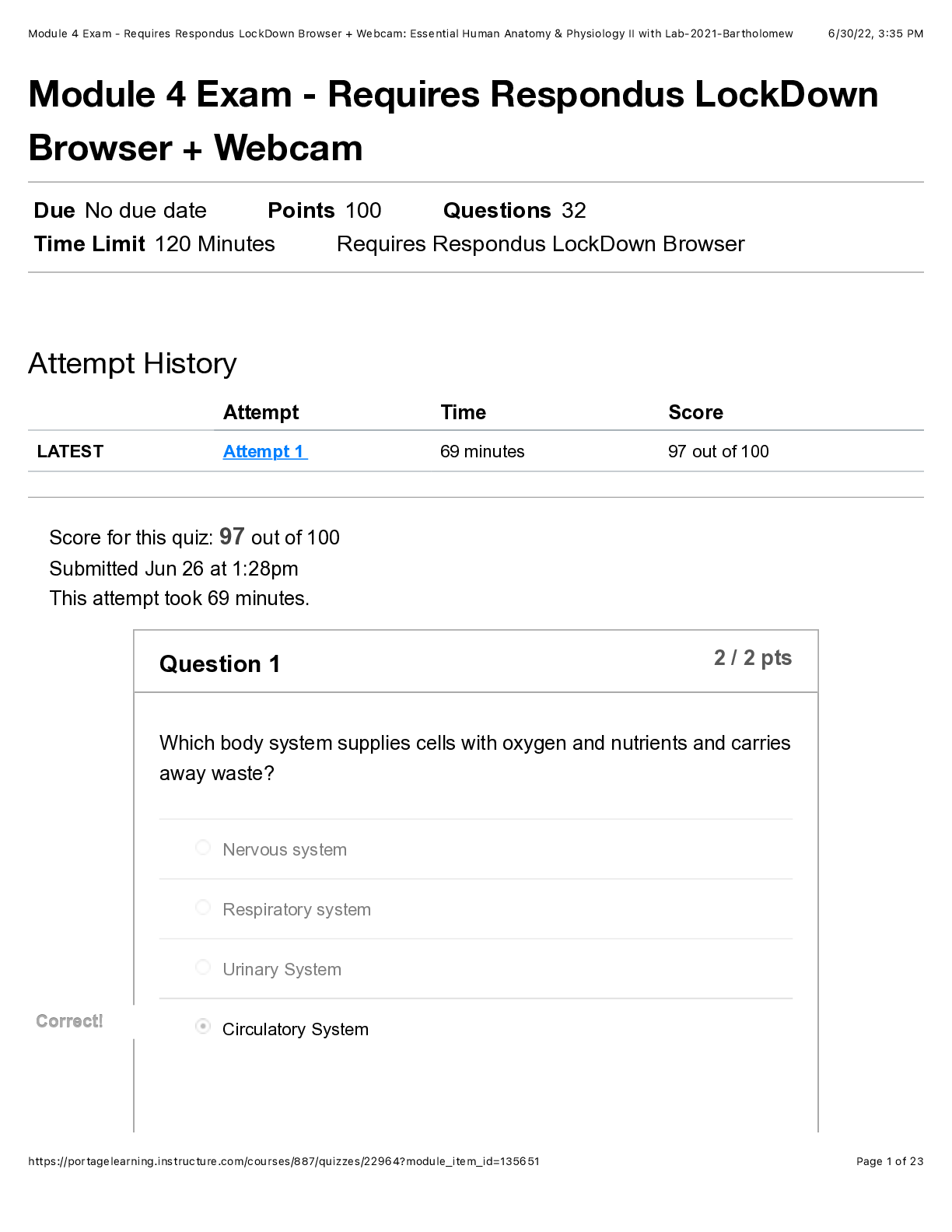
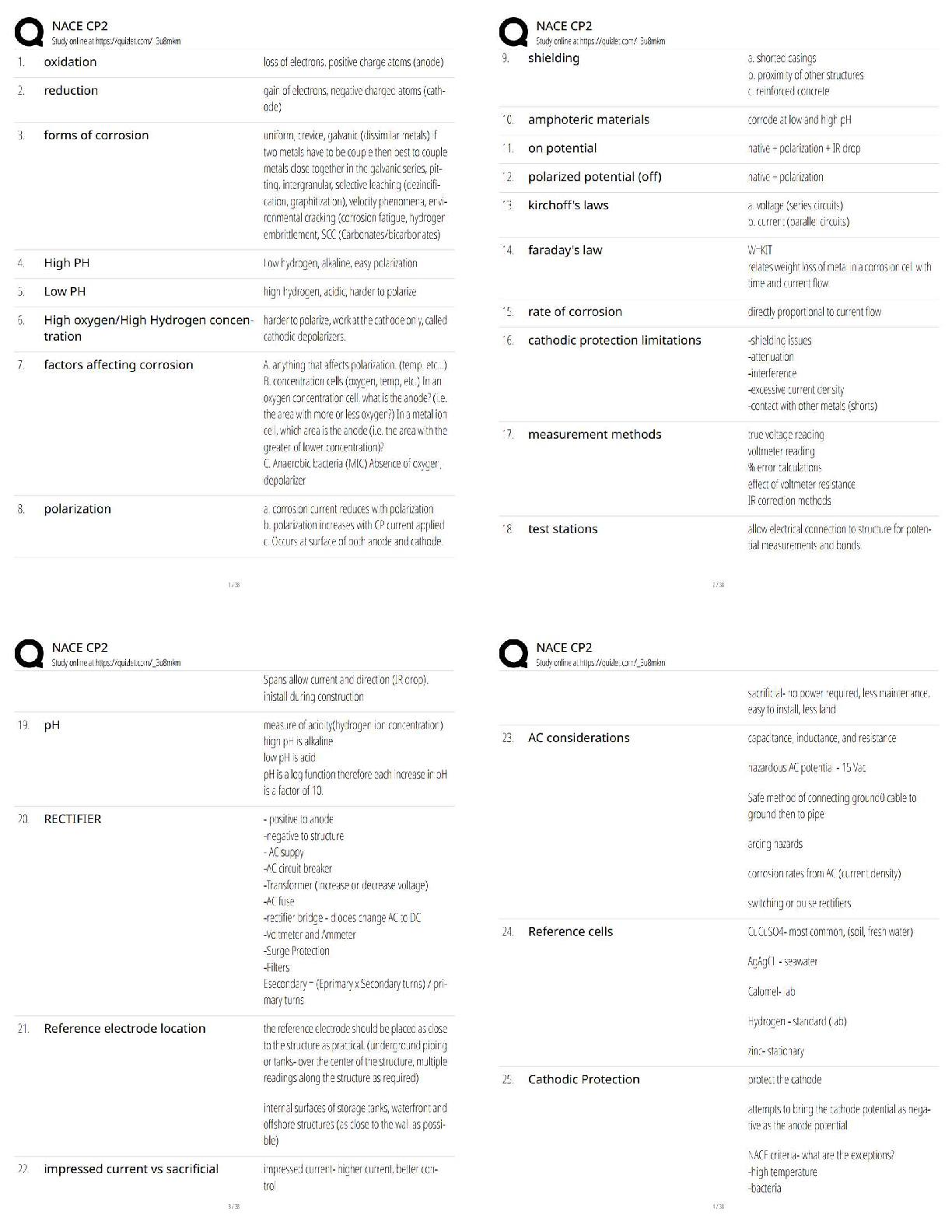
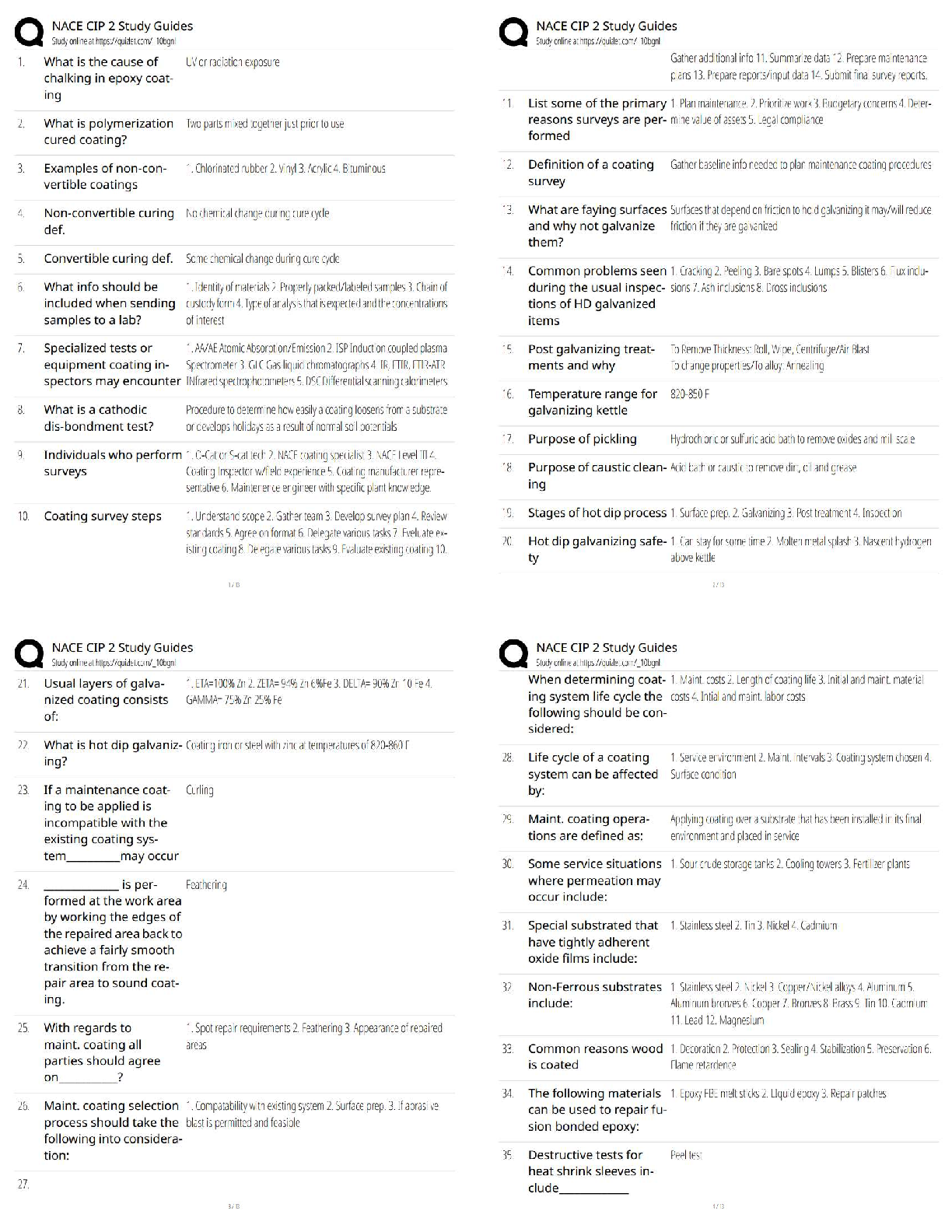
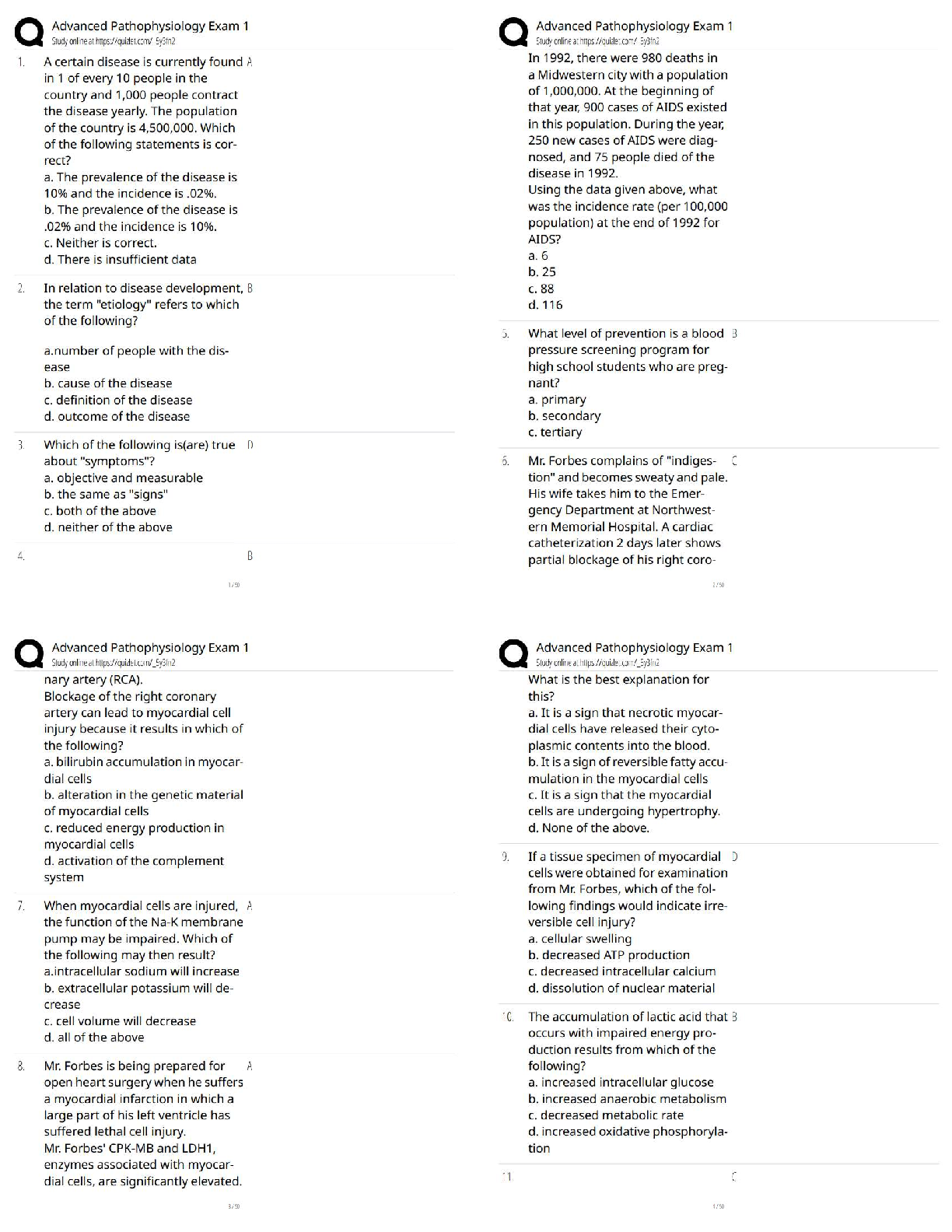
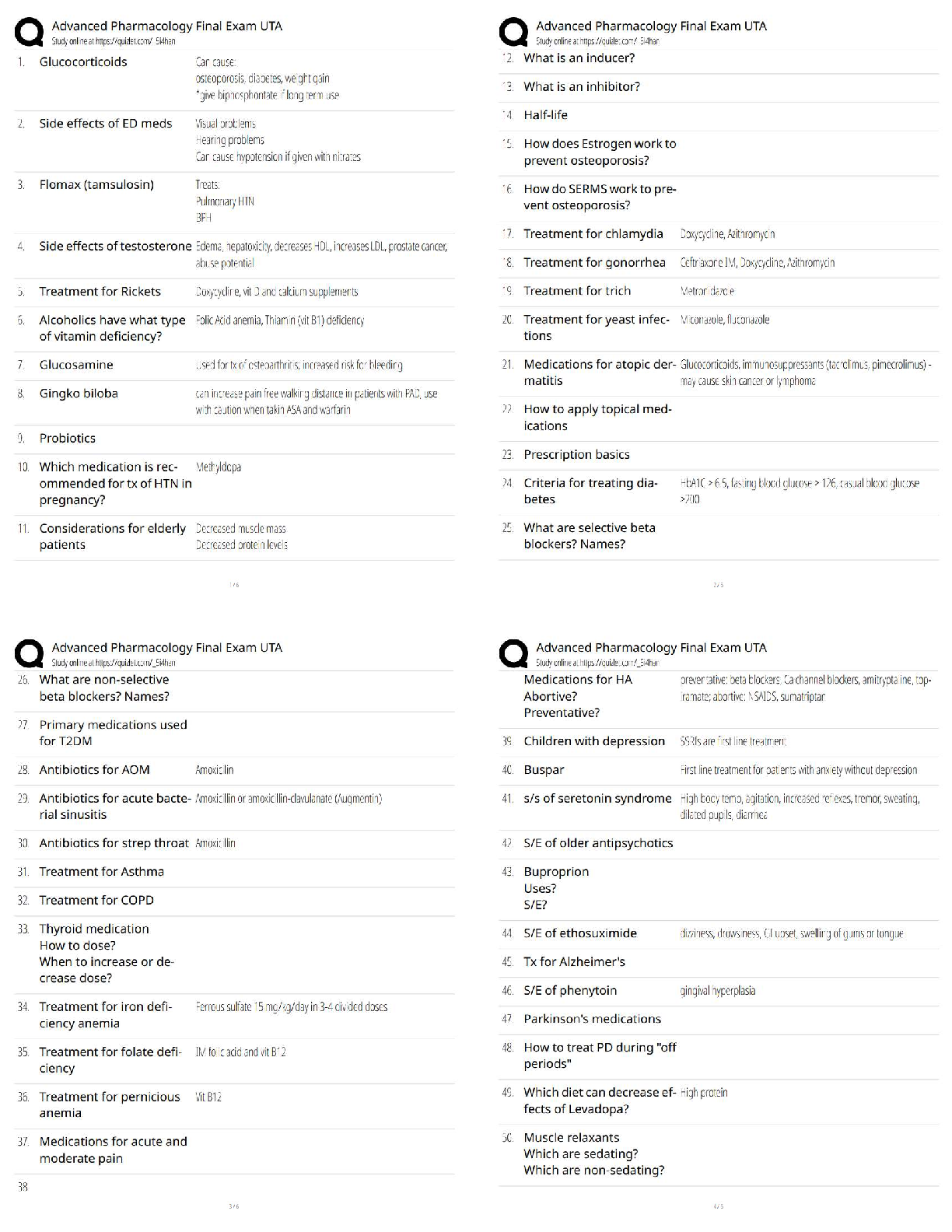
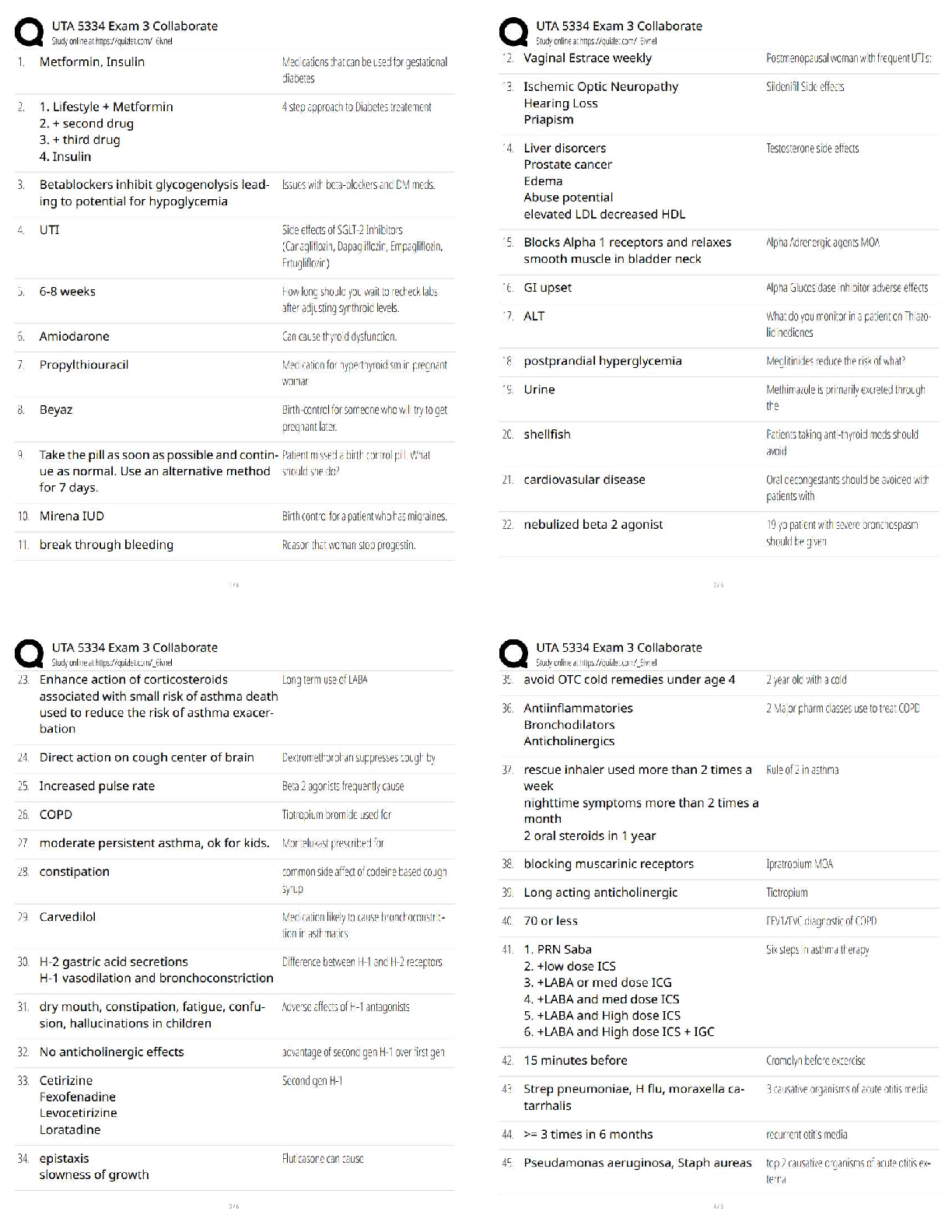
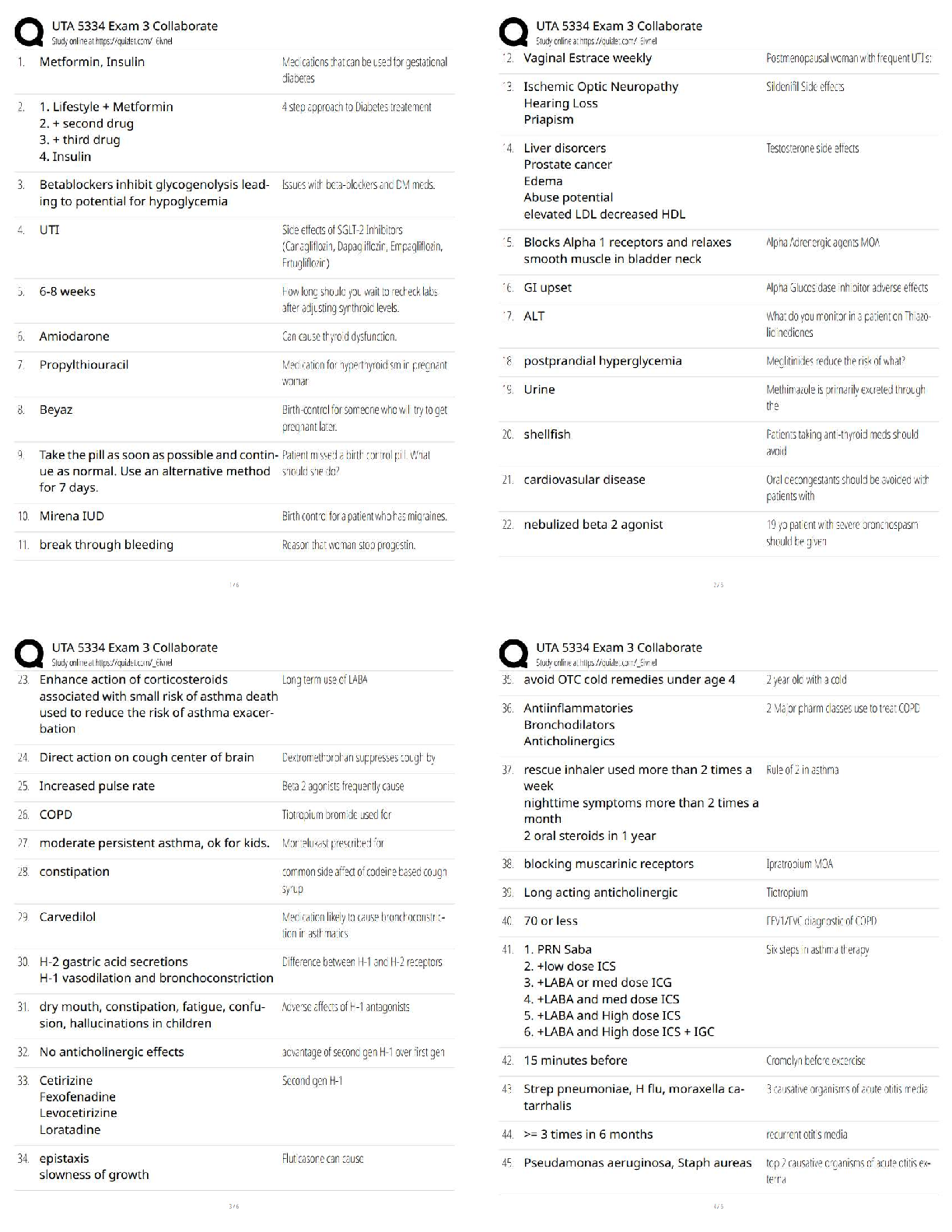
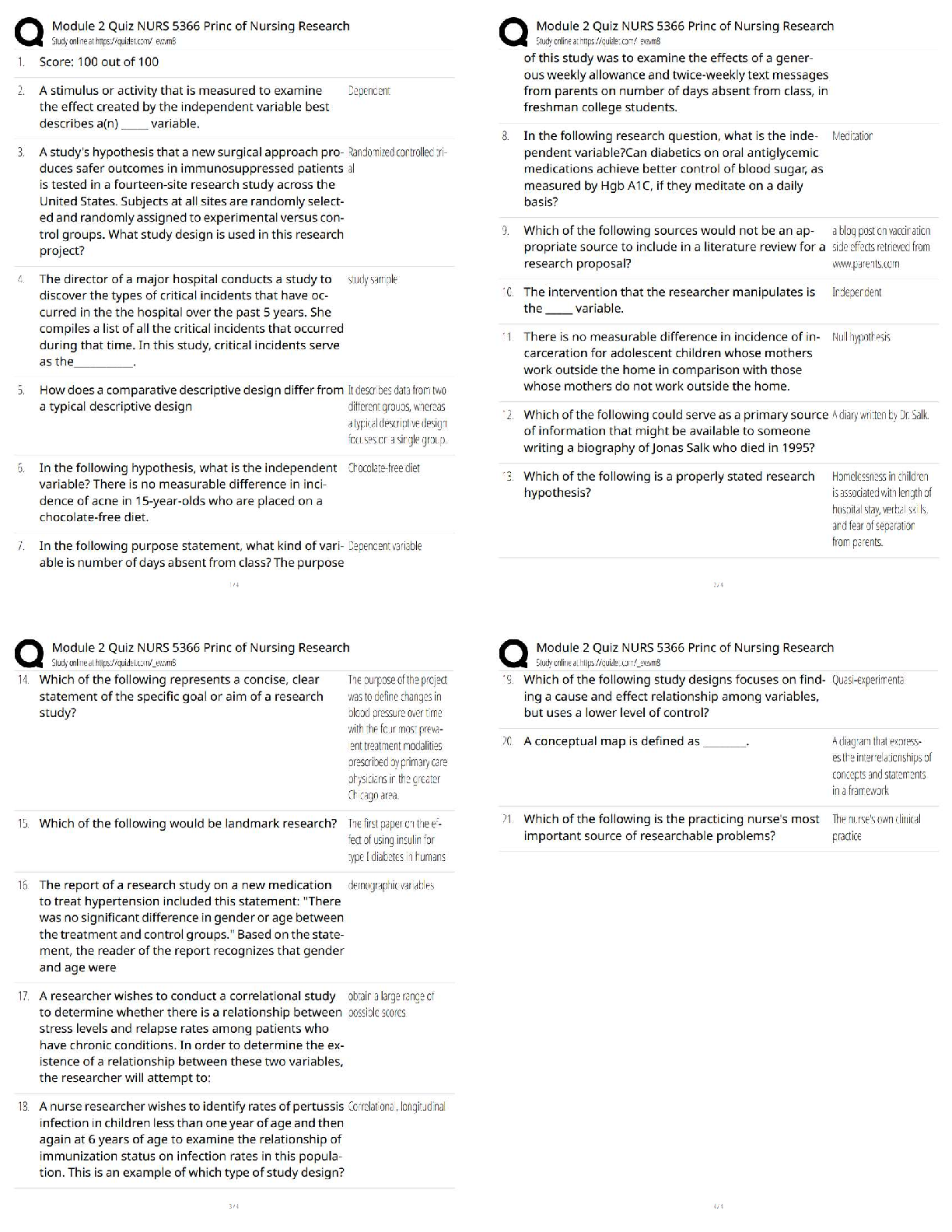
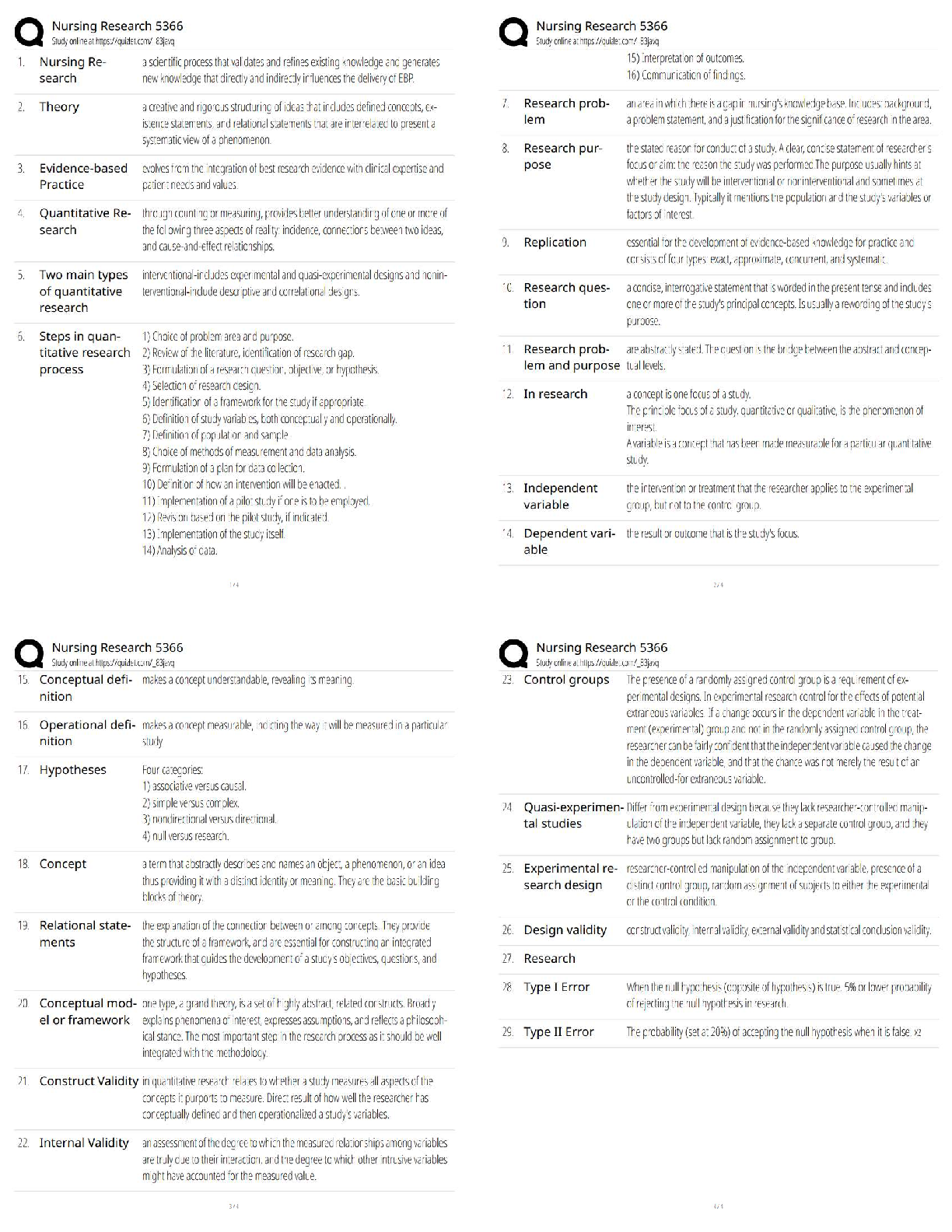
All of the following are considered “weak” interactions in proteins, except:
A) hydrogen bonds.
B) hydrophobic interactions.
C) ionic bonds.
D) peptide bonds.
E) van der Waals forces.
2. Overview of protein structu
...
All of the following are considered “weak” interactions in proteins, except:
A) hydrogen bonds.
B) hydrophobic interactions.
C) ionic bonds.
D) peptide bonds.
E) van der Waals forces.
2. Overview of protein structure
Pages: 114115 Difficulty: 2 Ans: A
The most important contribution to the stability of a protein’s conformation appears to be the:
A) entropy increase from the decrease in ordered water molecules forming a solvent shell around it.
B) maximum entropy increase from ionic interactions between the ionized amino acids in a protein.
C) sum of free energies of formation of many weak interactions among the hundreds of amino acids
in a protein.
D) sum of free energies of formation of many weak interactions between its polar amino acids and
surrounding water.
E) stabilizing effect of hydrogen bonding between the carbonyl group of one peptide bond and the
amino group of another.
3. Overview of protein structure
Page: 115 Difficulty: 1 Ans: D
In an aqueous solution, protein conformation is determined by two major factors. One is the
formation of the maximum number of hydrogen bonds. The other is the:
A) formation of the maximum number of hydrophilic interactions.
B) maximization of ionic interactions.
C) minimization of entropy by the formation of a water solvent shell around the protein.
D) placement of hydrophobic amino acid residues within the interior of the protein.
E) placement of polar amino acid residues around the exterior of the protein
[Show More]





 (1).png)