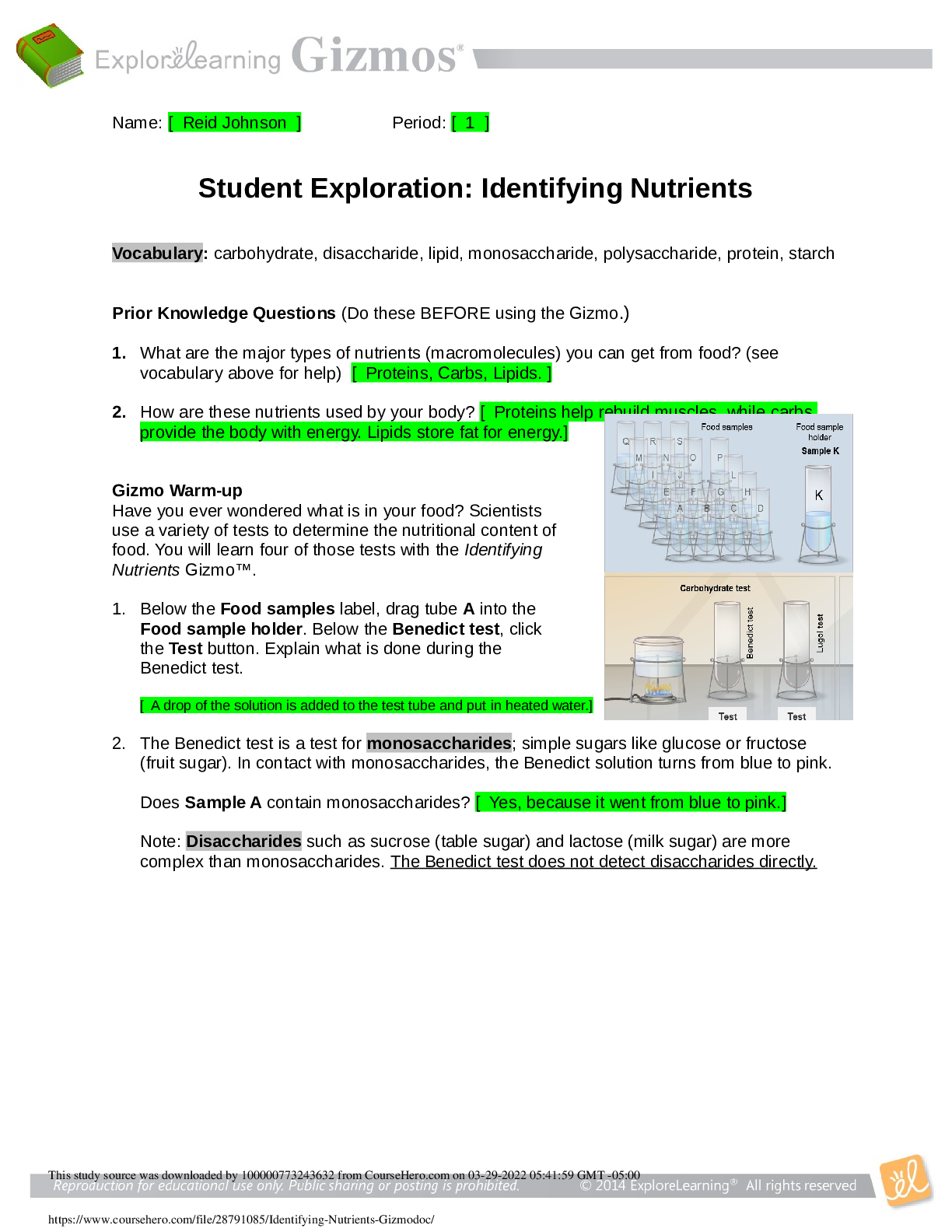
Vocabulary: covalent bond, diatomic molecule, Lewis diagram, molecule, noble gases,
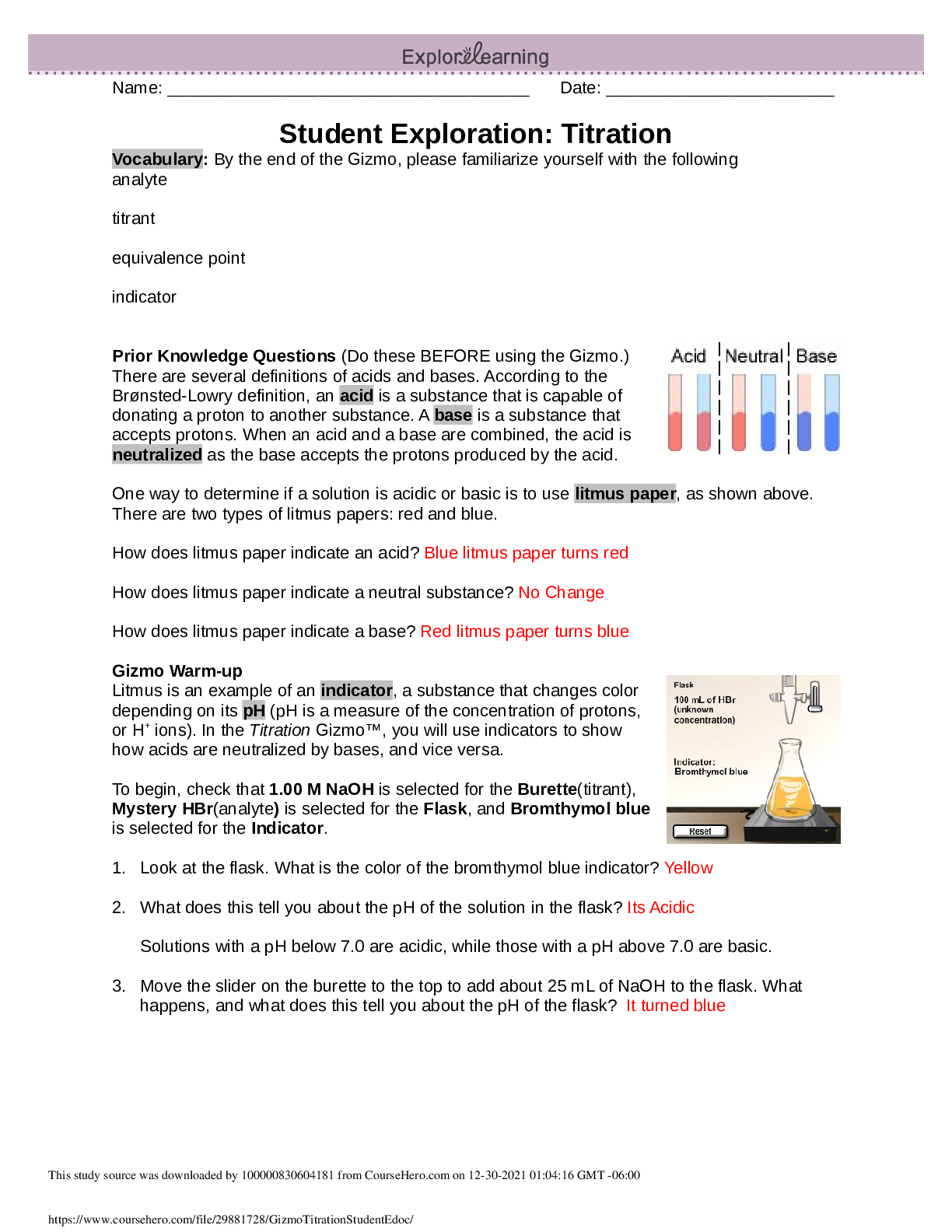
nonmetal, octet rule, shell, valence, valence electron
Prior Knowledge Questions (Do these BEFORE using the Gizmo.)
[Note: The purpos
...
Vocabulary: covalent bond, diatomic molecule, Lewis diagram, molecule, noble gases,
nonmetal, octet rule, shell, valence, valence electron
Prior Knowledge Questions (Do these BEFORE using the Gizmo.)
[Note: The purpose of these questions is to activate prior knowledge and get students thinking.
Students are not expected to know the answers to the Prior Knowledge Questions.]
1. There are eight markers in a full set, but Flora and Frank each only have seven markers.
Flora is missing the red marker, and Frank is missing the blue marker.
What can they do so that each has a full set of markers?
Frank can share his red marker with Flora, and Flora can share her blue marker with Frank.
2. Otto and Olivia each have six markers. Otto is missing the purple and green markers, and
Olivia is missing the black and brown markers. What can they do so that each has a full set?
Olivia can share her purple and green markers with Otto, and Otto can share his black and
brown markers with Olivia.
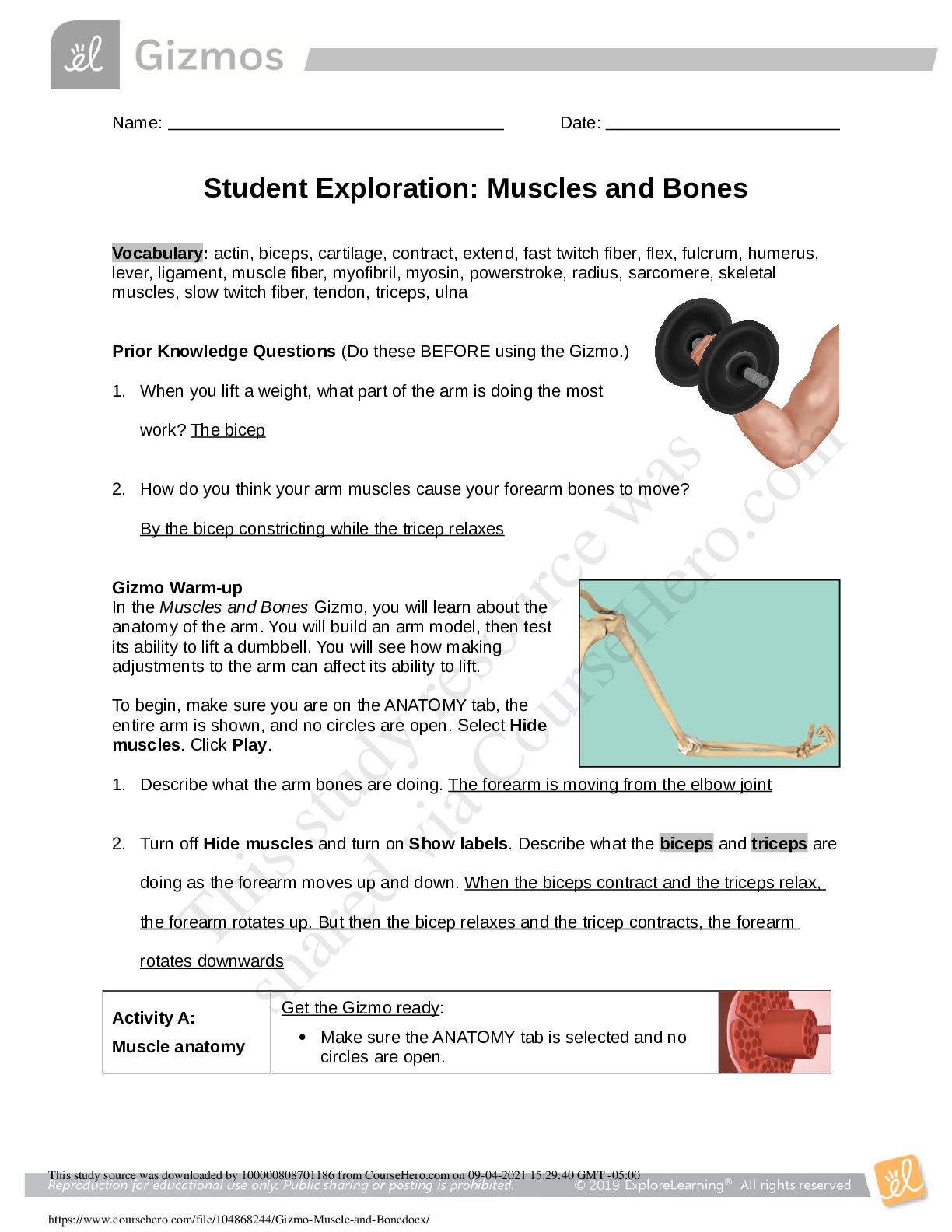
Gizmo Warm-up
Just like the students described above, nonmetal atoms
can share electrons. As you will see in the Covalent
Bonds Gizmo™, atoms form bonds in this way.
To begin, check that Fluorine is selected from the
Select a substance menu. Click Play ( ) to see the
electrons orbiting the nucleus of each atom.
1. The outermost electrons in each atom are called valence electrons. How many valence
electrons does each fluorine atom have? Each fluorine atom has seven valence electrons.
2. Click Pause ( ). Drag an electron from the left atom to the right atom. Click Play.
What happens? The electron that was dragged now orbits both atoms.
3. Click Pause, drag an electron from the right atom to the left, and then click Play.
[Show More]



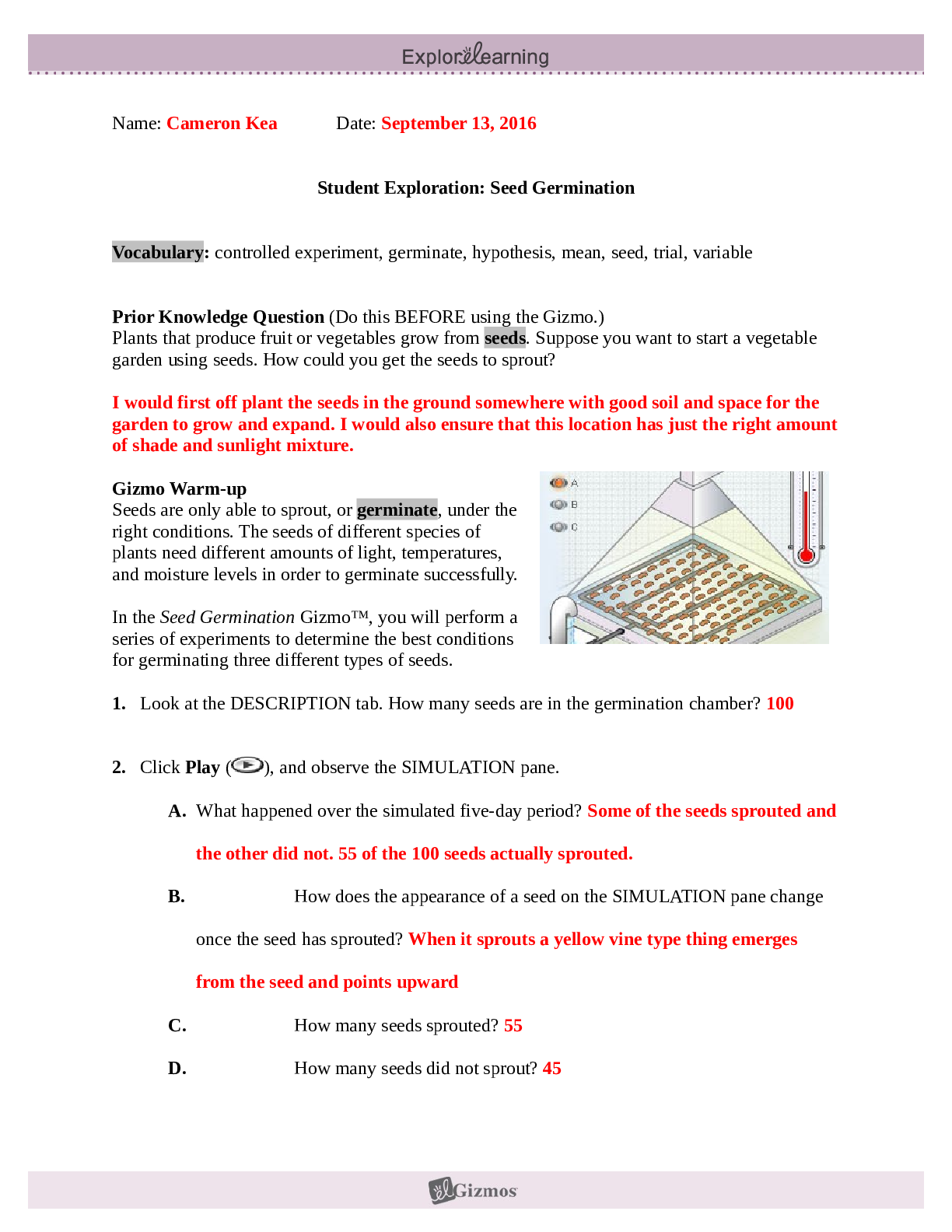

.png)
.png)
.png)

.png)
.png)