Question 1
2 out of 2 points
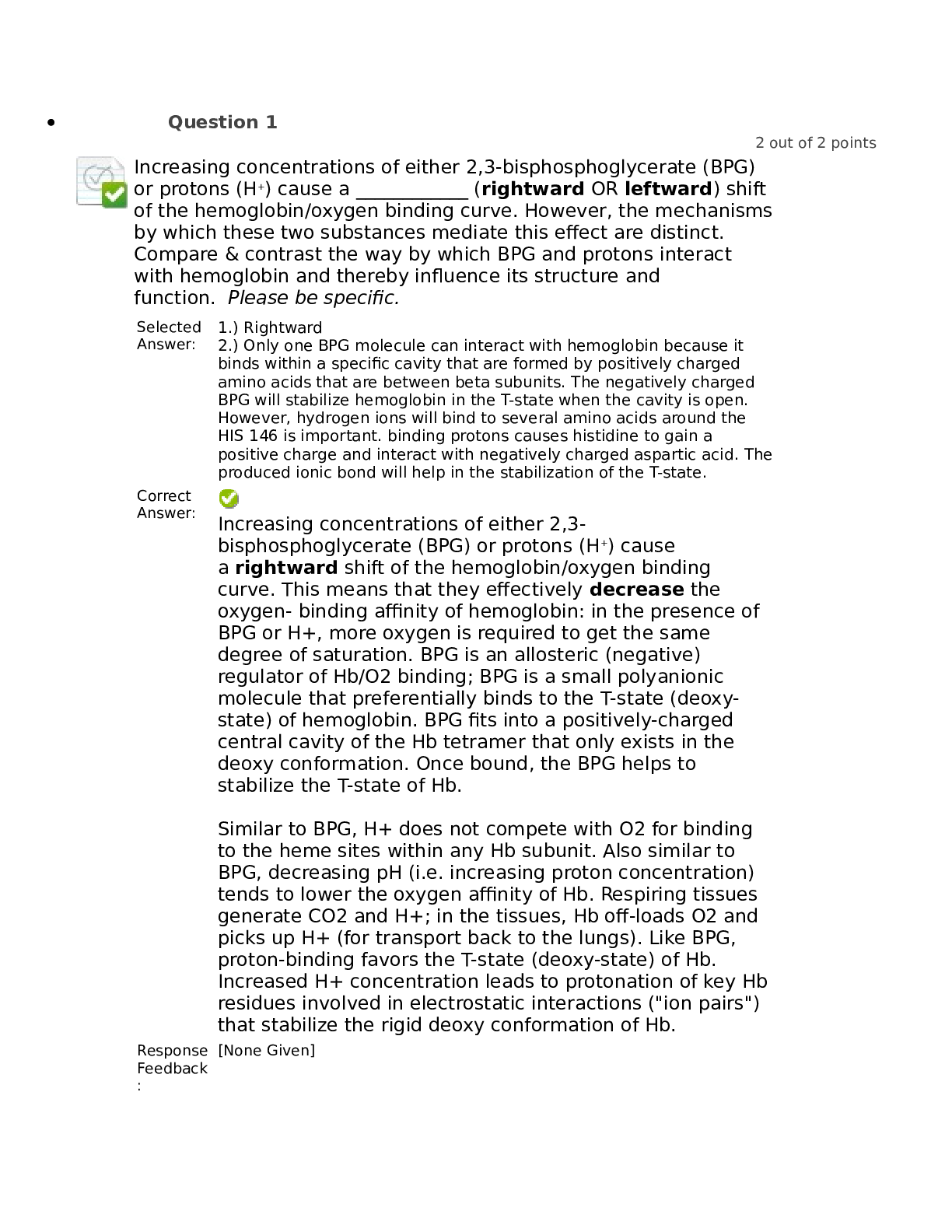
Increasing concentrations of either 2,3-bisphosphoglycerate (BPG)
or protons (H+) cause a ____________ (rightward OR leftward) shift
of the hemoglobin/oxygen binding curve. However, the m
...
Question 1
2 out of 2 points
Increasing concentrations of either 2,3-bisphosphoglycerate (BPG)
or protons (H+) cause a ____________ (rightward OR leftward) shift
of the hemoglobin/oxygen binding curve. However, the mechanisms
by which these two substances mediate this effect are distinct.
Compare & contrast the way by which BPG and protons interact
with hemoglobin and thereby influence its structure and
function. Please be specific.
Selected
Answer:
1.) Rightward
2.) Only one BPG molecule can interact with hemoglobin because it
binds within a specific cavity that are formed by positively charged
amino acids that are between beta subunits. The negatively charged
BPG will stabilize hemoglobin in the T-state when the cavity is open.
However, hydrogen ions will bind to several amino acids around the
HIS 146 is important. binding protons causes histidine to gain a
positive charge and interact with negatively charged aspartic acid. The
produced ionic bond will help in the stabilization of the T-state.
Correct
Answer:
Increasing concentrations of either 2,3-
bisphosphoglycerate (BPG) or protons (H+) cause
a rightward shift of the hemoglobin/oxygen binding
curve. This means that they effectively decrease the
oxygen- binding affinity of hemoglobin: in the presence of
BPG or H+, more oxygen is required to get the same
degree of saturation. BPG is an allosteric (negative)
regulator of Hb/O2 binding; BPG is a small polyanionic
molecule that preferentially binds to the T-state (deoxystate) of hemoglobin. BPG fits into a positively-charged
central cavity of the Hb tetramer that only exists in the
deoxy conformation. Once bound, the BPG helps to
stabilize the T-state of Hb.
Similar to BPG, H+ does not compete with O2 for binding
to the heme sites within any Hb subunit. Also similar to
BPG, decreasing pH (i.e. increasing proton concentration)
tends to lower the oxygen affinity of Hb. Respiring tissues
generate CO2 and H+; in the tissues, Hb off-loads O2 and
picks up H+ (for transport back to the lungs). Like BPG,
proton-binding favors the T-state (deoxy-state) of Hb.
Increased H+ concentration leads to protonation of key Hb
residues involved in electrostatic interactions ("ion pairs")
that stabilize the rigid deoxy conformation of Hb.
Response
Feedback
:
[None Given] Question 2
0.5 out of 0.5 points
Which of the following statements is true? Select any/all answers that apply.
Selected
Answers: D.
A single Mb molecule and a single Hb subunit have similar
tertiary structures.
Answers: A.
Both Mb and Hb bind O2 cooperatively.
B.
Mb and Hb have similar affinities for BPG (2,3-
bisphosphoglycerate).
C.
Both Mb and Hb transport oxygen within the blood.
D.
A single Mb molecule and a single Hb subunit have similar
tertiary structures.
E.
Both Mb and Hb contain multiple oxygen-binding sites.
Question 3
0.5 out of 0.5 points
At a pO2 of ~100 torr, hemoglobin in whole blood is about 90%
saturated with oxygen. This corresponds to ______________ pressure,
where the ____________ of hemoglobin is favored.
[Show More]




.png)

.png)
.png)
.png)


.png)