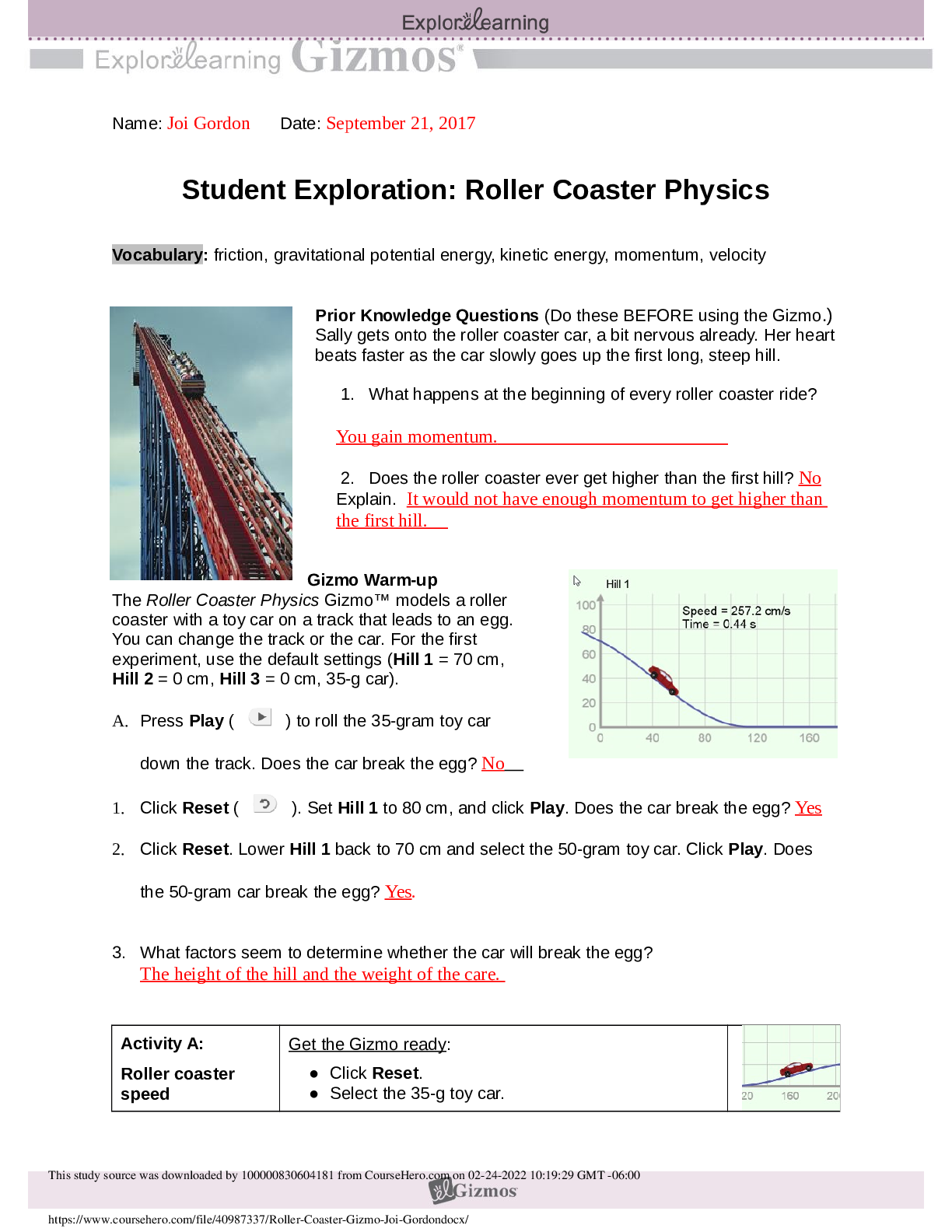
Student Exploration: Stoichiometry
Directions: Follow the instructions to go through the simulation. Respond to the questions and
prompts in the orange boxes.
Vocabulary: Avogadro’s number, balanced equation, cancel,
...
Student Exploration: Stoichiometry
Directions: Follow the instructions to go through the simulation. Respond to the questions and
prompts in the orange boxes.
Vocabulary: Avogadro’s number, balanced equation, cancel, coefficient, conversion factor, dimensional
analysis, molar mass, mole, molecular mass, stoichiometry
Prior Knowledge Questions (Do these BEFORE using the Gizmo.)
1. A 250 mL glass of orange juice contains 22 grams of sugar. How much sugar is in a two-liter (2,000 mL)
2. It requires two sticks of butter to make a batch of 20 cookies. How much butter will it take to make 150
Gizmo Warm-up
Just as a cook follows a recipe to decide how much of each
ingredient to add, a chemist uses stoichiometry to determine
the amounts of substances involved in chemical reactions. The
Stoichiometry Gizmo allows you to try your hand at figuring out
the amounts of reactants and products that take part in a
chemical reaction.
To begin, check that this equation is shown:
Fe
2O3 + 3CO 2Fe + 3CO2
1. Look at the coefficients (such as the “3” in 3CO) in front of each substance in the equation. The
coefficients tell you how many molecules or atoms take part in a chemical reaction. In the spaces below, list
the number of each molecule or atom in the equation:
2. In a balanced equation, the same number of each kind of atom is shown on each side of the equation.
Calculate the number of iron (Fe), oxygen (O), and carbon atoms (C).
Reproduction for educational use only. Public sharing or posting prohibited. © 2020 ExploreLearning™ All rights reserved
Name: Date:
bottle of orange juice? 176
cookies? 15 sticks
Fe
2O3 1 CO 3 Fe 2 CO2 3
Reactants Iron: 2 Oxygen: 6 Carbon: 3
Products Iron: 2 Oxygen: 6 Carbon: 3
Based on these values, is the equation balanced? yes
[Show More]