Chemistry > Lab Report > Bomb Calorimetry: Determination of the Resonance Energy of Benzene LAB REPORT (All)
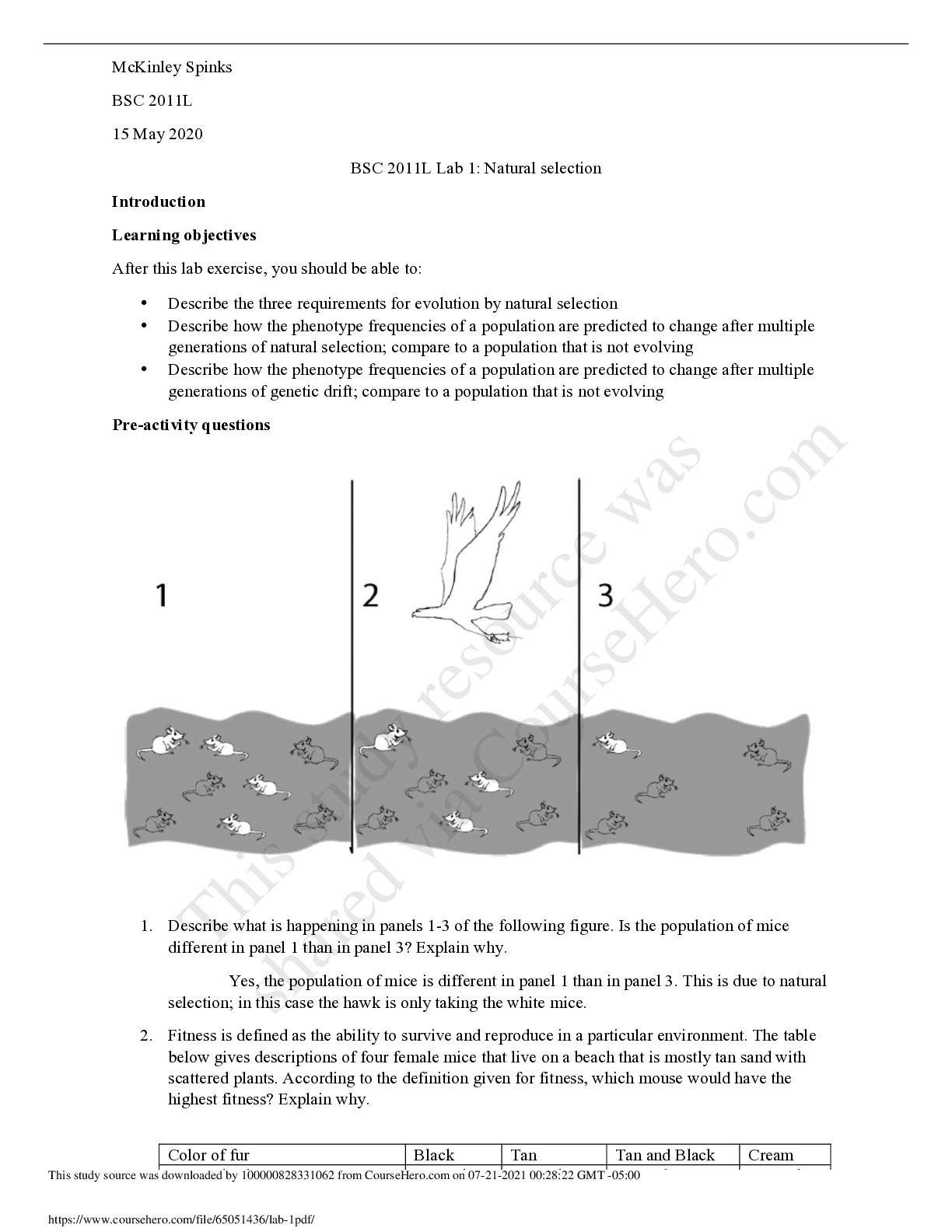
Bomb Calorimetry: Determination of the Resonance Energy of Benzene LAB REPORT
Document Content and Description Below
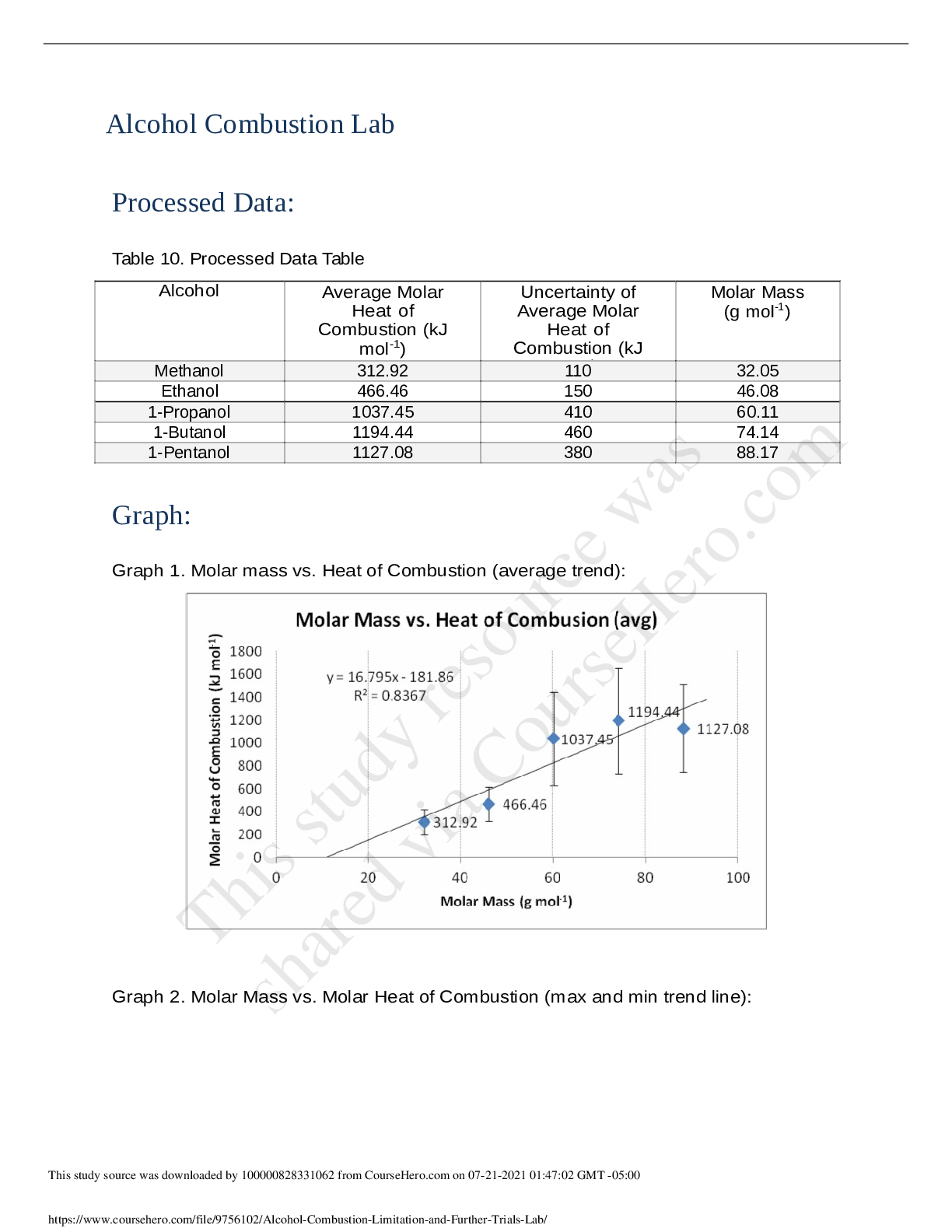
Name: Jiayi Sun Partner: Yuting LuoAbstract Objective: To determine the resonance energy of benzene from thermochemical data. Results collected: • The heat equivalent of the bomb calorimeter (b enzo... ic acid used) = 10422 ± 156 Joules • The molar heat (standard) of combustion of CDDT after correction: 7361 14 kJ/mol • The heat of combustion of benzene: -3267 kJ/mol (literature value) • The heat of vaporization of benzene: 30.72 kJ/mol (literature value) • The heat of combustion of cyclohexane: -3930 kJ/mol (literature value) • The heat of vaporization of cyclohexane: 33.334 kJ/mol (literature value) • The heat of vaporization of CDDT: -67.2 kJ/mol (literature value) • The resonance energy of benzene: 161 kJ/mol • Mole ratio of O2 to benzoic acid by using ideal gas law: n= 0.365 moles •Theory • In this experiment, the heat of combustion of CDDT (trans, trans, cis: liquid) was measured in the bomb calorimeter. • The combustion reaction of CDDT:• CDDT contains 3 C-C double bonds, 9 C-C single bonds, six C-H bonds, and six CH2 groups. Benzene posses 3 C-C double bonds, 3 C-C single bonds, and 6 C-H bonds; cyclohexane consists 6 C-C bonds and six CH2 groups. The bond total of benzene and cyclohexane equals to the number of bonds in CDDT. [Show More]
Last updated: 2 years ago
Preview 1 out of 19 pages

Buy this document to get the full access instantly
Instant Download Access after purchase
Buy NowInstant download
We Accept:

Reviews( 0 )
$9.00
Can't find what you want? Try our AI powered Search
Document information
Connected school, study & course
About the document
Uploaded On
Jul 20, 2021
Number of pages
19
Written in
Additional information
This document has been written for:
Uploaded
Jul 20, 2021
Downloads
0
Views
72